Abstract
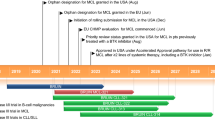
Acerta Pharma is developing the Bruton’s tyrosine kinase inhibitor acalabrutinib (Calquence®) for the treatment of various haematological and solid malignancies. The drug has received accelerated approval from the US FDA for the treatment of mantle cell lymphoma based on the results of a phase II study, and phase III trials in mantle cell lymphoma and chronic lymphocytic leukaemia are currently underway. This article summarizes the milestones in the development of acalabrutinib leading to this first approval for mantle cell lymphoma.
Similar content being viewed by others
References
AstraZeneca, Acerta Pharma. Acalabrutinib granted breakthrough therapy designation by US FDA for the treatment of patients with mantle cell lymphoma [media release]. 1 Aug 2017. http://www.acerta-pharma.com.
FDA. FDA approves new treatment for adults with mantle cell lymphoma [media release]. 31 Oct 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm583076.htm.
Acerta Pharma. Acerta Pharma announces strategic transaction with AstraZeneca [media release]. 17 Dec 2015. http://www.acerta-pharma.com.
Harrington BK, Gulrajani M, Covey T, et al. ACP-196 is a second generation inhibitor of bruton tyrosine kinase (BTK) with enhanced target specificity [abstract]. Blood. 2015;126(23):2908.
Covey T, Barf T, Gulrajani M, et al. ACP-196: a novel covalent Bruton’s tyrosine kinase (Btk) inhibitor with improved selectivity and in vivo target coverage in chronic lymphocytic leukemia (CLL) patients [abstract no. 2596]. Cancer Res. 2015;75(15 Suppl).
Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323–32.
Herman SEM, Montraveta A, Niemann CU, et al. The Bruton Tyrosine Kinase (BTK) inhibitor acalabrutinib demonstrates potent on-target effects and efficacy in two mouse models of chronic lymphocytic leukemia. Clin Cancer Res. 2017;23(11):2831–41.
Harrington BK, Gardner HL, Izumi R, et al. Preclinical evaluation of the novel BTK inhibitor acalabrutinib in canine models of B-cell non-Hodgkin lymphoma. PLoS ONE. 2016;11(7):e0159607.
AstraZeneca. CALQUENCE® (acalabrutinib): prescribing information. 2017. https://www.azpicentral.com/calquence/calquence.pdf#page=1. Accessed 2017.
Wang M, Rule SA, Zinzani PL, et al. Efficacy and safety of acalabrutinib monotherapy in patients with relapsed/refractory mantle cell lymphoma in the phase 2 ACE-LY-004 study [abstract no. 155]. In: American Society of Hematology (ASH) 59th Annual Meeting and Exposition. 2017.
Awan FT, Schuh A, Brown JR, et al. Acalabrutinib monotherapy in patients with ibrutinib intolerance: results from the phase 1/2 ACE-CL-001 clinical study [abstract]. Blood. 2016;128(22):638.
Hillmen P, Schuh A, Eyre TA, et al. Acalabrutinib monotherapy in patients with richter transformation from the phase 1/2 ACE-CL-001 clinical study [abstract]. Blood. 2016;128(22):60.
Byrd J, Wierda W, Schuh A, et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: updated results from the phase 1/2 ACE-CL-001 study [abstract no. 498]. In: American Society of Hematology (ASH) 59th Annual Meeting and Exposition. 2017.
Wierda W, Jones J, Furman R, et al. Acalabrutinib, a second-generation bruton tyrosine kinase (BTK) inhibitor, in previously untreated chronic lymphocytic leukemia (CLL) [abstract no. S431]. Haematologica. 2016;101(Suppl 1):151.
Woyach J, Awan F, Jianfar M, et al. Acalabrutinib with obinutuzumab in relapsed/refractory and treatment-naive patients with chronic lymphocytic leukemia: the phase 1b/2 ACE-CL-003 study [abstract no. 432]. In: American Society of Hematology (ASH) 59th Annual Meeting and Exposition. 2017.
Overman MJ, Lopez CD, Benson AB, et al. A randomized phase 2 study of the Bruton tyrosine kinase (Btk) inhibitor acalabrutinib alone or with pembrolizumab for metastatic pancreatic cancer (mPC) [abstract no. 4130]. J Clin Oncol. 2016;34(15 Suppl).
Zhang T, Harrison M, O’Donnell P, et al. Phase 2 study of pembrolizumab alone or combined with acalabrutinib in platinum-refractory metastatic urothelial carcinoma (mUC) [abstract no. 863P]. Ann Oncol. 2017;28(Suppl 5):303–4.
Byrd J, Owen R, O’Brien S, et al. Pooled Analysis of Safety Data from Clinical Trials Evaluating Acalabrutinib Monotherapy in Hematologic Malignancies [abstract no. 4326]. In: American Society of Hematology (ASH) 59th Annual Meeting and Exposition. 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. A. Markham is a contracted employee and S. Dhillon is a salaried employee of Adis/Springer. They are responsible for the article content, and declare no relevant conflicts of interest.
Additional information about this Adis Drug Review can be found at http://www.medengine.com/Redeem/24FCF060581038CF.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Markham, A., Dhillon, S. Acalabrutinib: First Global Approval. Drugs 78, 139–145 (2018). https://doi.org/10.1007/s40265-017-0852-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0852-8