Abstract
Background
There are very few options to treat multidrug-resistant bacterial infections in children. A major barrier is the duration and complexity of regulatory trials of new antibiotics. Extrapolation of safety data from adult trials could facilitate drug development for children.
Objective
We performed a systematic review on the safety of antibiotic clinical trials (CTs) in children (0–18 years) to evaluate the overall quality of safety trials conducted in children and to determine if age-specific adverse events (AEs) could be identified for specific antibiotic classes.
Data Sources
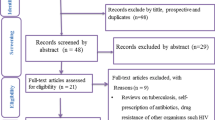
We searched the MEDLINE, Cochrane CENTRAL, and ClinicalTrials.gov electronic databases for trials conducted between 2000 and 2016.
Study Selection
All trials in which safety was declared a primary or secondary endpoint were included. Exclusion criteria were (1) topical or inhalational route of administration; (2) non-infectious conditions; (3) administration for prophylaxis rather than treatment; (4) selected population (i.e. cystic fibrosis, malignancies, HIV and tuberculosis); and (5) design other than randomized controlled trials. Trials reporting data on both adults and children were included only if paediatric results were reported separately.
Data Extraction and Synthesis
Two authors independently extracted the data. To assess the quality of published trials, the Extension for harms for Consolidated Standards of Reporting Trials (CONSORT) Statement 2004 was used.
Main Outcome and Measure
In order to quantitatively assess the rate of developing AEs by drug class, the numbers of overall and body-system-specific AEs were collected for each study arm, and then calculated per single drug class as median and interquartile range (IQR) of the proportions across CTs. The AEs most frequently reported were compared in the meta-analysis by selecting the CTs on the most represented drug classes.
Results
Eighty-three CTs were included, accounting for 27,693 children. Overall, 69.7% of CONSORT items were fully reported. The median proportion of children with any AE was 22.5%, but did not exceed 8% in any single body system. Serious drug-related AEs and drug-related discontinuations were very rare (median 0.3 and 0.9%, respectively). Limitations included the inability to stratify by age group, particularly neonates.
Conclusions and Relevance
Overall, AEs in paediatric antibiotic CTs were predictable and class-specific, and no unexpected (age-specific) side effects were identified. Smaller, open-label, dose-finding, high-quality, single-arm pharmacokinetic trials seem potentially sufficient for certain common antibiotic classes, extrapolating well-established safety profiles determined from large adult efficacy trials. This approach could reduce duration and enhance subsequent registration of urgently needed new antibiotics. This will need to be combined with enhanced methods of pharmacovigilance for monitoring of emerging AEs in routine clinical practice.



Similar content being viewed by others
References
Guidance for Industry. The Content and Format for Pediatric Use Supplements. Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). 1996. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071957.pdf. Accessed 15 Feb 2017.
European Medicines Agency. The European paediatric initiative: History of the Paediatric Regulation (EMEA/17967/04 Rev 1). 2007. http://www.ema.europa.eu/docs/en_GB/document_library/Other/2009/09/WC500003693.pdf. Accessed 15 Feb 2017.
Folgori L, Bielicki J, Ruiz B, et al. Harmonisation in study design and outcomes in paediatric antibiotic clinical trials: a systematic review. Lancet Infect Dis. 2016;16(9):e178–89.
European Medicines Agency. Concept paper on an addendum to the guideline on the evaluation of medicinal products indicated for treatment of bacterial infections (CPMP/EWP/558/95 rev 2) to address paediatric-specific clinical data requirements (EMA/CHMP/213862/2016). 2016. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/04/WC500205026.pdf. Accessed 15 Feb 2017.
Clinical Trials Transformation Initiative. Improving pediatric trials in antibacterial drug development: no sick child left behind. Summary of the Meeting held April 5, 2016. https://www.ctti-clinicaltrials.org/files/ctti-pedstrials-expertmeeting-summary.pdf. Accessed 15 Feb 2017.
Clinical Trials Transformation Initiative. CTTI recommendations: improving pediatric trials in antibacterial drug development. 2017. https://www.ctti-clinicaltrials.org/sites/www.ctti-clinicaltrials.org/files/abdd-pedstrials-recs.pdf. Accessed 15 Feb 2017.
European Medicines Agency. Work plan for the Modelling and Simulation Working Group (MSWG) for 2017 (EMA/799154/2016). 2016. http://www.ema.europa.eu/docs/en_GB/document_library/Work_programme/2016/04/WC500205035.pdf. Accessed 14 Nov 2017.
European Medicines Agency. Reflection paper on the use of extrapolation in the development of medicines for paediatrics. (EMA/199678/2016) Draft 2016. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/10/WC500236640.pdf. Accessed 14 Nov 2017.
Osokogu OU, Dodd C, Pacurariu A, Kaguelidou F, Weibel D, Sturkenboom MC. Drug Safety Monitoring in Children: performance of signal detection algorithms and impact of age stratification. Drug Saf. 2016;39(9):873–81.
European Medicines Agency. Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections (CPMP/EWP/558/95 rev 2). 2011. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003417.pdf. Accessed 15 Feb 2017.
Osokogu OU, Dukanovic J, Ferrajolo C, et al. Pharmacoepidemiological safety studies in children: a systematic review. Pharmacoepidemiol Drug Saf. 2016;25(8):861–70.
US Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.0. 2014. http://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf. Accessed 15 Feb 2017.
IND safety reporting. e-CFR (ELECTRONIC CODE OF FEDERAL REGULATIONS). Title 21, Chapter I, Subchapter D, Part 312, Subpart B, 312.32. https://www.ecfr.gov/cgi-bin/text-idx?SID=f70b2100e4501ebcba76f4cc0bb4afea&mc=true&node=se21.5.312_132&rgn=div8. Accessed 15 Feb 2017.
Ioannidis JP, Evans SJ, Gotzsche PC, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141(10):781–8.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41.
Tshefu A, Lokangaka A, African Neonatal Sepsis Trial group, et al. Oral amoxicillin compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with fast breathing when referral is not possible: a randomised, open-label, equivalence trial. Lancet. 2015;385(9979):1758–66.
Tshefu A, Lokangaka A, African Neonatal Sepsis Trial group, et al. Simplified antibiotic regimens compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with clinical signs of possible serious bacterial infection when referral is not possible: a randomised, open-label, equivalence trial. Lancet. 2015;385(9979):1767–76.
Baqui AH, Saha SK, Ahmed AS, et al. Safety and efficacy of alternative antibiotic regimens compared with 7 day injectable procaine benzylpenicillin and gentamicin for outpatient treatment of neonates and young infants with clinical signs of severe infection when referral is not possible: a randomised, open-label, equivalence trial. Lancet Glob Health. 2015;3(5):e279–87.
Chong CY, Tan AS, Ng W, Tan-Kendrick A, Balakrishnan A, Chao SM. Treatment of urinary tract infection with gentamicin once or three times daily. Acta Paediatr. 2003;92(3):291–6.
Deville JG, Adler S, Azimi PH, et al. Linezolid versus vancomycin in the treatment of known or suspected resistant gram-positive infections in neonates. Pediatr Infect Dis J. 2003;22(9 Suppl):S158–63.
Guadalupe Vásquez-Mendoza M, Vargas-Origel A, Carmen Ramos-Jiménez A, Aguilar-Orozco G, Romero-Gutiérrez G. Efficacy and renal toxicity of one daily dose of amikacin versus conventional dosage regime. Am J Perinatol. 2007;24(2):141–6.
Perez V, Saenz D, Madriz J, et al. A double-blind study of the efficacy and safety of multiple daily doses of amikacin versus one daily dose for children with perforated appendicitis in Costa Rica. Int J Infect Dis. 2011;15(8):e569–75.
Uijtendaal EV, Rademaker CM, Schobben AF, et al. Once-daily versus multiple-daily gentamicin in infants and children. Ther Drug Monit. 2001;23(5):506–13.
Yellin AE, Johnson J, Higareda I, et al. Ertapenem or ticarcillin/clavulanate for the treatment of intra-abdominal infections or acute pelvic infections in pediatric patients. Am J Surg. 2007;194(3):367–74.
Evaluation of safety, pharmacokinetics and efficacy of CAZ-AVI with metronidazole in children aged 3 months to 18 years old With Complicated Intra-abdominal Infections (cIAIs). ClinicalTrials.gov Identifier: NCT02475733. https://clinicaltrials.gov/ct2/show/NCT02475733?term=NCT02475733&rank=1. Accessed 20 Jan 2017.
Safety and efficacy study of ceftaroline versus a comparator in pediatric subjects with complicated skin infections. ClinicalTrials.gov Identifier: NCT01400867. https://clinicaltrials.gov/ct2/show/NCT01400867?term=NCT01400867&rank=1. Accessed 20 Jan 2017.
Safety and efficacy study of ceftaroline versus a comparator in pediatric subjects with Community Acquired Bacterial Pneumonia (CABP). ClinicalTrials.gov Identifier: NCT01530763. https://clinicaltrials.gov/ct2/show/NCT01530763?term=NCT01530763&rank=1. Accessed 20 Jan 2017.
Safety and efficacy study of ceftaroline versus a comparator in pediatric subjects with Complicated Community Acquired Pneumonia (CABP). ClinicalTrials.gov Identifier: NCT01669980. https://clinicaltrials.gov/ct2/show/NCT01669980?term=nCT01669980&rank=1. Accessed 20 Jan 2017.
Safety and efficacy of solithromycin in adolescents and children with community-acquired bacterial pneumonia. ClinicalTrials.gov Identifier: NCT02605122. https://clinicaltrials.gov/ct2/show/NCT02605122?term=NCT02605122&rank=1. Accessed 20 Jan 2017.
Comparative evaluation of the safety and efficacy of daptomycin versus SOC in 1–17 Year Olds With Staphylococcus Aureus Bacteremia (MK-3009-005). ClinicalTrials.gov Identifier: NCT01728376. https://clinicaltrials.gov/ct2/show/NCT01728376?term=NCT01728376&rank=1. Accessed 20 Jan 2017.
Study of tedizolid phosphate in adolescents with Complicated Skin and Soft Tissue Infection (cSSTI) (MK-1986-012). ClinicalTrials.gov Identifier: NCT02276482. https://clinicaltrials.gov/ct2/show/NCT02276482?term=NCT02276482&rank=1. Accessed 20 Jan 2017.
Evaluation of Safety, Pharmacokinetics and Efficacy of Ceftazidime and Avibactam (CAZ-AVI) compared with cefepime in children from 3 months to less than 18 years of age with complicated Urinary Tract Infections (cUTIs). ClinicalTrials.gov Identifier: NCT02497781. https://clinicaltrials.gov/ct2/show/NCT02497781?term=NCT02497781&rank=1. Accessed 20 Jan 2017.
Abdel-Hady E, Hamamsy M, Hedaya M, Awad H. The efficacy and toxicity of two dosing-regimens of amikacin in neonates with sepsis. J Clin Pharm Ther. 2011;36(1):45–52.
Adler M, McDonald PJ, Trostmann U, Keyserling C, Tack K. Cefdinir vs. amoxicillin/clavulanic acid in the treatment of suppurative acute otitis media in children. Pediatr Infect Dis J. 2000;19(12 Suppl):S166–70.
Aguilar A, Tinoco JC, Macias M, et al. Clinical and bacteriologic efficacy of amoxycillin b.d. (45 mg/kg/day) versus amoxycillin t.d.s (40 mg/kg/day) in children with group A beta-hemolytic streptococcal tonsillopharyngitis. J Chemother. 2000;12(5):396–405.
Arguedas A, Emparanza P, Schwartz RH, et al. A randomized, multicenter, double blind, double dummy trial of single dose azithromycin versus high dose amoxicillin for treatment of uncomplicated acute otitis media. Pediatr Infect Dis J. 2005;24(2):153–61.
Arguedas A, Soley C, Kamicker BJ, Jorgensen DM. Single-dose extended-release azithromycin versus a 10-day regimen of amoxicillin/clavulanate for the treatment of children with acute otitis media. Int J Infect Dis. 2011;15(4):e240–8.
Arrieta A, Arguedas A, Fernandez P, et al. High-dose azithromycin versus high-dose amoxicillin-clavulanate for treatment of children with recurrent or persistent acute otitis media. Antimicrob Agents Chemother. 2003;47(10):3179–86.
Balatsouras DG, Korres S, Rallis E, Eliopoulos P, Ferekidis E. Twice-daily dosing of loracarbef 15 mg/kg versus 30 mg/kg in the treatment of children with acute sinusitis. Drugs Exp Clin Res. 2005;31(Suppl):1–5.
Baysoy G, Saltik Temizel IN, Uslu N, et al. Ornidazole-based sequential therapy is not effective in Helicobacter pylori eradication in children. Turk J Gastroenterol. 2013;24(5):382–6.
Begum B, Haque MA, Ahmed MS, et al. Comparison between azithromycin and cefixime in the treatment of typhoid fever in children. Mymensingh Med J. 2014;23(3):441–8.
Block SL, McCarty JM, Hedrick JA, et al. Comparative safety and efficacy of cefdinir vs amoxicillin/clavulanate for treatment of suppurative acute otitis media in children. Pediatr Infect Dis J. 2000;19(12 Suppl):S159–65.
Block SL, Schmier JK, Notario GF, et al. Efficacy, tolerability, and parent reported outcomes for cefdinir vs. high-dose amoxicillin/clavulanate oral suspension for acute otitis media in young children. Curr Med Res Opin. 2006;22(9):1839–47.
Boccazzi A, Tonelli P, De’Angelis M, Bellussi L, Passali D, Careddu P. Short course therapy with cefitbuten versus azithromycin in pediatric streptococcal pharyngitis. Pediatr Infect Dis J. 2000;19(10):963–7.
Bradley JS, Arguedas A, Blumer JL, Saez-Llorens X, Melkote R, Noel GJ. Comparative study of levofloxacin in the treatment of children with community-acquired pneumonia. Pediatr Infect Dis J. 2007;26(10):868–78.
Carapetis JR, Jaquiery AL, Buttery JP, et al. Randomized, controlled trial comparing once daily and three times daily gentamicin in children with urinary tract infections. Pediatr Infect Dis J. 2001;20(3):240–6.
Cascio A, Colomba C, Antinori S, Paterson DL, Titone L. Clarithromycin versus azithromycin in the treatment of Mediterranean spotted fever in children: a randomized controlled trial. Clin Infect Dis. 2002;34(2):154–8.
Cascio A, Colomba C, Di Rosa D, Salsa L, di Martino L, Titone L. Efficacy and safety of clarithromycin as treatment for Mediterranean spotted fever in children: a randomized controlled trial. Clin Infect Dis. 2001;33(3):409–11.
Chanta C, Phloenchaiwanit P. Randomized controlled trial of azithromycin versus doxycycline or chloramphenicol for treatment of uncomplicated pediatric scrub typhus. J Med Assoc Thai. 2015;98(8):756–60.
Chotigeat U, Narongsanti A, Ayudhya DP. Gentamicin in neonatal infection: once versus twice daily dosage. J Med Assoc Thai. 2001;84(8):1109–15.
Cochereau I, Goldschmidt P, Goepogui A, et al. Efficacy and safety of short duration azithromycin eye drops versus azithromycin single oral dose for the treatment of trachoma in children: a randomised, controlled, double-masked clinical trial. Br J Ophthalmol. 2007;91(5):667–72.
Cohen R, Reinert P, De La Rocque F, et al. Comparison of two dosages of azithromycin for three days versus penicillin V for ten days in acute group A streptococcal tonsillopharyngitis. Pediatr Infect Dis J. 2002;21(4):297–303.
Damrikarnlert L, Jauregui AC, Kzadri M. Efficacy and safety of amoxycillin/clavulanate (Augmentin) twice daily versus three times daily in the treatment of acute otitis media in children. The Augmentin 454 Study Group. J Chemother. 2000;12(1):79–87.
Demirjian A, Finkelstein Y, Nava-Ocampo A, et al. A randomized controlled trial of a vancomycin loading dose in children. Pediatr Infect Dis J. 2013;32(11):1217–23.
English M, Mohammed S, Ross A, et al. A randomised, controlled trial of once daily and multi-dose daily gentamicin in young Kenyan infants. Arch Dis Child. 2004;89(7):665–9.
Eppes SC, Childs JA. Comparative study of cefuroxime axetil versus amoxicillin in children with early Lyme disease. Pediatrics. 2002;109(6):1173–7.
Esposito S, Marchisio P, Bosis S, et al. Comparative efficacy and safety of 5-day cefaclor and 10-day amoxycillin treatment of group A streptococcal pharyngitis in children. Int J Antimicrob Agents. 2002;20(1):28–33.
Ferwerda A, Moll HA, Hop WC, et al. Efficacy, safety and tolerability of 3 day azithromycin versus 10 day co-amoxiclav in the treatment of children with acute lower respiratory tract infections. J Antimicrob Chemother. 2001;47(4):441–6.
Haczynski J, Chmielik M, Bien S, et al. A comparative study of cefaclor vs amoxicillin/clavulanate in pediatric pharyngotonsillitis. Med Sci Monit. 2003;9(3):I29–35.
Jantausch BA, Deville J, Adler S, et al. Linezolid for the treatment of children with bacteremia or nosocomial pneumonia caused by resistant gram-positive bacterial pathogens. Pediatr Infect Dis J. 2003;22(9 Suppl):S164–71.
Kafetzis DA, Liapi G, Tsolia M, et al. Failure to eradicate Group A beta-haemolytic streptococci (GABHS) from the upper respiratory tract after antibiotic treatment. Int J Antimicrob Agents. 2004;23(1):67–71.
Kafetzis DA, Maltezou HC, Mavrikou M, et al. Isepamicin versus amikacin for the treatment of acute pyelonephritis in children. Int J Antimicrob Agents. 2000;14(1):51–5.
Kaplan SL, Deville JG, Yogev R, et al. Linezolid versus vancomycin for treatment of resistant Gram-positive infections in children. Pediatr Infect Dis J. 2003;22(8):677–86.
Khan AM, Ahmed T, Alam NH, Chowdhury AK, Fuchs GJ. Extended-interval gentamicin administration in malnourished children. J Trop Pediatr. 2006;52(3):179–84.
Langley JM, Halperin SA, Boucher FD, Smith B, Pediatric Investigators Collaborative Network on Infections in C. Azithromycin is as effective as and better tolerated than erythromycin estolate for the treatment of pertussis. Pediatrics. 2004;114(1):e96–101.
Lebel MH, Mehra S. Efficacy and safety of clarithromycin versus erythromycin for the treatment of pertussis: a prospective, randomized, single blind trial. Pediatr Infect Dis J. 2001;20(12):1149–54.
Lee PI, Wu MH, Huang LM, Chen JM, Lee CY. An open, randomized, comparative study of clarithromycin and erythromycin in the treatment of children with community-acquired pneumonia. J Microbiol Immunol Infect. 2008;41(1):54–61.
Marild S, Jodal U, Sandberg T. Ceftibuten versus trimethoprim-sulfamethoxazole for oral treatment of febrile urinary tract infection in children. Pediatr Nephrol. 2009;24(3):521–6.
McCarty J, Hedrick JA, Gooch WM. Clarithromycin suspension vs penicillin V suspension in children with streptococcal pharyngitis. Adv Ther. 2000;17(1):14–26.
Nizic T, Velikanje E, Ruzic-Sabljic E, Arnez M. Solitary erythema migrans in children: comparison of treatment with clarithromycin and amoxicillin. Wien Klin Wochenschr. 2012;124(13–14):427–33.
Noel GJ, Blumer JL, Pichichero ME, et al. A randomized comparative study of levofloxacin versus amoxicillin/clavulanate for treatment of infants and young children with recurrent or persistent acute otitis media. Pediatr Infect Dis J. 2008;27(6):483–9.
Pareek A, Kulkarni M, Daga S, Deshpande A, Chandurkar N. Comparative evaluation of efficacy and safety of cefotaxime-sulbactam with amoxicillin-clavulanic acid in children with lower respiratory tract infections. Expert Opin Pharmacother. 2008;9(16):2751–7.
Pichichero ME, Gooch WM 3rd. Comparison of cefdinir and penicillin V in the treatment of pediatric streptococcal tonsillopharyngitis. Pediatr Infect Dis J. 2000;19(12 Suppl):S171–3.
Poachanukoon O, Kitcharoensakkul M. Efficacy of cefditoren pivoxil and amoxicillin/clavulanate in the treatment of pediatric patients with acute bacterial rhinosinusitis in Thailand: a randomized, investigator-blinded, controlled trial. Clin Ther. 2008;30(10):1870–9.
Portier H, Bourrillon A, Lucht F, et al. Treatment of acute group A beta-hemolytic streptococcal tonsillitis in children with a 5-day course of josamycin. Arch Pediatr. 2001;8(7):700–6.
Saez-Llorens X, McCoig C, Feris JM, et al. Quinolone treatment for pediatric bacterial meningitis: a comparative study of trovafloxacin and ceftriaxone with or without vancomycin. Pediatr Infect Dis J. 2002;21(1):14–22.
Sakata H. Comparative study of 5-day cefcapene–pivoxil and 10-day amoxicillin or cefcapene–pivoxil for treatment of group A streptococcal pharyngitis in children. J Infect Chemother. 2008;14(3):208–12.
Shahid SK. Efficacy and safety of cefepime in late-onset ventilator-associated pneumonia in infants: a pilot randomized and controlled study. Ann Trop Med Parasitol. 2008;102(1):63–71.
Sher L, Arguedas A, Husseman M, et al. Randomized, investigator-blinded, multicenter, comparative study of gatifloxacin versus amoxicillin/clavulanate in recurrent otitis media and acute otitis media treatment failure in children. Pediatr Infect Dis J. 2005;24(4):301–8.
Tiwari S, Rehan HS, Chandra J, Mathur NN, Singh V. Efficacy and safety of a single daily dose of gentamicin in hospitalized Indian children: a quasi-randomized trial. J Antimicrob Chemother. 2009;64(5):1096–101.
Wang CY, Lu CY, Hsieh YC, Lee CY, Huang LM. Intramuscular ceftriaxone in comparison with oral amoxicillin–clavulanate for the treatment of acute otitis media in infants and children. J Microbiol Immunol Infect. 2004;371:57–62.
Wible K, Tregnaghi M, Bruss J, Fleishaker D, Naberhuis-Stehouwer S, Hilty M. Linezolid versus cefadroxil in the treatment of skin and skin structure infections in children. Pediatr Infect Dis J. 2003;22(4):315–23.
Yogev R, Patterson LE, Kaplan SL, et al. Linezolid for the treatment of complicated skin and skin structure infections in children. Pediatr Infect Dis J. 2003;22(9 Suppl):S172–7.
Zimbabwe Bangladesh, South Africa (Zimbasa) Dysentery Study Group. Multicenter, randomized, double blind clinical trial of short course versus standard course oral ciprofloxacin for Shigella dysenteriae type 1 dysentery in children. Pediatr Infect Dis J. 2002;21(12):1136–41.
Trial on the ideal duration of oral antibiotics in children with pneumonia. ClinicalTrials.gov Identifier: NCT02258763. https://clinicaltrials.gov/ct2/show/NCT02258763?term=NCT02258763&rank=1. Accessed 20 Jan 2017.
Short-course Antimicrobial Therapy for Paediatric Respiratory Infections (SAFER). ClinicalTrials.gov Identifier: NCT02380352. https://clinicaltrials.gov/ct2/show/NCT02380352?term=NCT02380352&rank=1. Accessed 20 Jan 2017.
Duration of antimicrobial therapy for paediatric pneumonia. ClinicalTrials.gov Identifier: NCT01707485. https://clinicaltrials.gov/ct2/show/NCT01707485?term=NCT01707485&rank=1. Accessed 20 Jan 2017.
Bolus Versus Prolonged Infusion of Meropenem in Newborn With Late Onset Sepsis (BVPIMNBLOS). ClinicalTrials.gov Identifier: NCT02503761. https://clinicaltrials.gov/ct2/show/NCT02503761?term=NCT02503761&rank=1. Accessed 20 Jan 2017.
Efficacy, Pharmacokinetics and Safety of Meropenem in Infants Below 90 Days With Clinical or Confirmed Late-onset Sepsis (NeoMero-1). ClinicalTrials.gov Identifier: NCT01551394. Available at: https://clinicaltrials.gov/ct2/show/NCT01551394?term=NCT01551394&rank=1. Accessed 20 Jan 2017.
Safety and efficacy study of daptomycin compared to active comparator in pediatric participants with Acute Hematogenous Osteomyelitis (AHO) (MK-3009-006). ClinicalTrials.gov Identifier: NCT01922011. https://clinicaltrials.gov/ct2/show/NCT01922011?term=NCT01922011&rank=1. Accessed 20 Jan 2017.
Antibiotic Safety (SCAMP). ClinicalTrials.gov Identifier: NCT01994993. https://clinicaltrials.gov/ct2/show/NCT01994993?term=NCT01994993&rank=1. Accessed 20 Jan 2017.
Efficacy of antibiotics in children with acute sinusitis: which subgroups benefit? ClinicalTrials.gov Identifier: NCT02554383. https://clinicaltrials.gov/ct2/show/NCT02554383?term=NCT02554383&rank=1. Accessed 20 Jan 2017.
Tailored therapy for Helicobacter pylori in children. ClinicalTrials.gov Identifier: NCT02635191. https://clinicaltrials.gov/ct2/show/NCT02635191?term=NCT02635191&rank=1. Accessed 20 Jan 2017.
Hospitalised Pneumonia With Extended Treatment (HOPE) Study (HOPE). ClinicalTrials.gov Identifier: NCT02783859. https://clinicaltrials.gov/ct2/show/NCT02783859?term=NCT02783859&rank=1. Accessed 20 Jan 2017.
Neonatal Vancomycin Trial (NeoVanc). ClinicalTrials.gov Identifier: NCT02790996. https://clinicaltrials.gov/ct2/show/NCT02790996?term=NCT02790996&rank=1. Accessed 20 Jan 2017.
Non-operative Management for Appendicitis in Children (APRES). ClinicalTrials.gov Identifier: NCT02795793. https://clinicaltrials.gov/ct2/show/NCT02795793?term=NCT02795793&rank=1. Accessed 20 Jan 2017.
Comparing the intravenous treatment of skin infections in children, Home Versus Hospital (CHOICE). ClinicalTrials.gov Identifier: NCT02334124. https://clinicaltrials.gov/ct2/show/NCT02334124?term=NCT02334124&rank=1. Accessed 20 Jan 2017.
Arguedas A, Cespedes J, Botet FA, et al. Safety and tolerability of ertapenem versus ceftriaxone in a double-blind study performed in children with complicated urinary tract infection, community-acquired pneumonia or skin and soft-tissue infection. Int J Antimicrob Agents. 2009;33(2):163–7.
Wargo KA, Edwards JD. Aminoglycoside-induced nephrotoxicity. J Pharm Pract. 2014;27(6):573–7.
Myers AL, Gaedigk A, Dai H, James LP, Jones BL, Neville KA. Defining risk factors for red man syndrome in children and adults. Pediatr Infect Dis J. 2012;31(5):464–8.
The Pew Charitable Trusts. Antibiotics currently in clinical development. 2016. http://www.pewtrusts.org/~/media/assets/2016/12/antibiotics_datatable_201612.pdf. Accessed 15 Feb 2017.
Khashab MM, Xiang J, Kahn JB. Comparison of the adverse event profiles of levofloxacin 500 mg and 750 mg in clinical trials for the treatment of respiratory infections. Curr Med Res Opin. 2006;22(10):1997–2006.
Wang Y, Zou Y, Xie J, et al. Linezolid versus vancomycin for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a systematic review employing meta-analysis. Eur J Clin Pharmacol. 2015;71(1):107–15.
Yuan X, Liang BB, Wang R, et al. Treatment of community-acquired pneumonia with moxifloxacin: a meta-analysis of randomized controlled trials. J Chemother. 2012;24(5):257–67.
Momper JD, Chang Y, Jackson M, et al. Adverse event detection and labeling in pediatric drug development. Ther Innov Regul Sci. 2015;49(2):302–9.
Adderson EE, Flynn PM, Hoffman JM. Efficacy and safety of cefepime in pediatric patients: a systematic review and meta-analysis. J Pediatr. 2010;157(3):490–5.
Adefurin A, Sammons H, Jacqz-Aigrain E, Choonara I. Ciprofloxacin safety in paediatrics: a systematic review. Arch Dis Child. 2011;96(9):874–80.
Ioannidou M, Apostolidou-Kiouti F, Haidich AB, Niopas I, Roilides E. Efficacy and safety of linezolid for the treatment of infections in children: a meta-analysis. Eur J Pediatr. 2014;173(9):1179–86.
Anderson M, Choonara I. A systematic review of safety monitoring and drug toxicity in published randomised controlled trials of antiepileptic drugs in children over a 10-year period. Arch Dis Child. 2010;95(9):731–8.
de Vries TW, van Roon EN. Low quality of reporting adverse drug reactions in paediatric randomised controlled trials. Arch Dis Child. 2010;95(12):1023–6.
Children’s Hospital Association. Pediatric Health Information System (PHIS). https://www.childrenshospitals.org/programs-and-services/data-analytics-and-research/pediatric-analytic-solutions/pediatric-health-information-system. Accessed 15 Sept 2017.
Bonhoeffer J, Kochhar S, Hirschfeld S, et al. Global alignment of immunization safety assessment in pregnancy—The GAIA project. Vaccine. 2016;34(49):5993–7.
McDonnell A, Rex JH, Goossens H, Bonten M, Fowler VG Jr, Dane A. Efficient delivery of investigational antibacterial agents via sustainable clinical trial networks. Clin Infect Dis. 2016;63(Suppl 2):S57–9.
Holzmann-Pazgal G, Khan AM, Northrup TF, Domonoske C, Eichenwald EC. Decreasing vancomycin utilization in a neonatal intensive care unit. Am J Infect Control. 2015;43(11):1255–7.
Mulhall A, de Louvois J, Hurley R. Chloramphenicol toxicity in neonates: its incidence and prevention. Br Med J (Clin Res Ed). 1983;287(6403):1424–7.
European Medicines Agency. Initiative for patient registries. Strategy and pilot phase. (EMA/176050/2014). 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Other/2015/10/WC500195576.pdf. Accessed 14 Nov 2017.
Author information
Authors and Affiliations
Contributions
MS and LF contributed to the concept and design of the study. MS, LF and PP designed the search strategy and selection criteria. PP and LF collected the data. All authors contributed to the interpretation of the data. PP, LF and MS wrote the first draft of the manuscript. All authors reviewed and contributed to subsequent drafts and approved the final version for publication. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The corresponding author confirms that he had full access to all the data in the study and had final responsibility for the decision to submit the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
Mike Sharland reported grants from GSK, Pfizer, and DNDi outside the submitted work. Paola Pansa, Yingfen Hsia, Julia Bielicki, Irja Lutsar, A. Sarah Walker, and Laura Folgori had no conflicts of interest to disclose.
Funding
This study did not receive any direct funding.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pansa, P., Hsia, Y., Bielicki, J. et al. Evaluating Safety Reporting in Paediatric Antibiotic Trials, 2000–2016: A Systematic Review and Meta-Analysis. Drugs 78, 231–244 (2018). https://doi.org/10.1007/s40265-017-0850-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0850-x