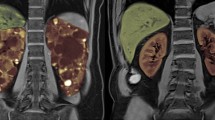
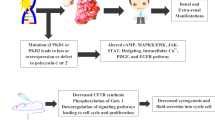
Abstract
Tolvaptan (Jinarc®) is a highly selective vasopressin V2 receptor antagonist indicated for use in patients with autosomal dominant polycystic kidney disease (ADPKD). Tolvaptan is the first pharmaceutical agent to be approved in Europe for delaying the progression of ADPKD in adults with stage 1–3 chronic kidney disease at initiation of treatment. In the large phase III TEMPO 3:4 trial in adults with ADPKD, 3 years’ treatment with oral tolvaptan significantly reduced growth in total kidney volume and slowed renal function decline relative to placebo. Tolvaptan was also associated with a significantly lower rate of events for the composite secondary endpoint of time to investigator-assessed clinical progression relative to placebo, an effect that was largely attributable to reductions in the risk of worsening renal function and the risk of worsening kidney pain. Many of the most common adverse events in the tolvaptan group were related to its aquaretic mechanism of action (e.g. polyuria, nocturia, polydipsia and thirst). Tolvaptan was also associated with idiosyncratic elevations of liver enzymes which were reversible on discontinuation of the drug. Although the use of tolvaptan requires careful consideration and balancing of benefits and risks, current evidence suggests that tolvaptan is a promising new treatment option for patients with ADPKD.

Similar content being viewed by others
References
Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369(9569):1287–301.
Chapman AB, Devuyst O, Eckardt KU, et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2015;88(1):17–27.
McGovern AP, Jones S, van Vlymen J, et al. Identification of people with autosomal dominant polycystic kidney disease using routine data: a cross sectional study. BMC Nephrol. 2014;15:182.
Neumann HP, Jilg C, Bacher J, et al. Epidemiology of autosomal-dominant polycystic kidney disease: an in-depth clinical study for south-western Germany. Nephrol Dial Transplant. 2013;28(6):1472–87.
Ars E, Bernis C, Fraga G, et al. Spanish guidelines for the management of autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2014;29(Suppl 4):iv95–105.
Cornec-Le Gall E, Audrezet MP, Chen JM, et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013;24(6):1006–13.
Wuthrich RP, Mei C. Pharmacological management of polycystic kidney disease. Expert Opin Pharmacother. 2014;15(8):1085–95.
European Medicines Agency. Tolvaptan (Samsca): summary of product characteristics. http://www.ema.europa.eu. Accessed 18 Sep 2015.
Reif GA, Yamaguchi T, Nivens E, et al. Tolvaptan inhibits ERK-dependent cell proliferation, Cl(−) secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am J Physiol Renal Physiol. 2011;301(5):F1005–13.
Meijer E, Gansevoort RT, de Jong PE, et al. Therapeutic potential of vasopressin V2 receptor antagonist in a mouse model for autosomal dominant polycystic kidney disease: optimal timing and dosing of the drug. Nephrol Dial Transplant. 2011;26(8):2445–53.
European Medicines Agency. Tolvaptan (Jinarc): summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 18 Sep 2015.
Aihara M, Fujiki H, Mizuguchi H, et al. Tolvaptan delays the onset of end-stage renal disease in a polycystic kidney disease model by suppressing increases in kidney volume and renal injury. J Pharmacol Exp Ther. 2014;349(2):258–67.
Shoaf SE, Wang Z, Bricmont P, et al. Pharmacokinetics, pharmacodynamics, and safety of tolvaptan, a nonpeptide AVP antagonist, during ascending single-dose studies in healthy subjects. J Clin Pharmacol. 2007;47(12):1498–507.
European Medicines Agency. Jinarc assessment report. 2015. http://www.ema.europa.eu. Accessed 18 Sep 2015.
Torres VE, Meijer E, Bae KT, et al. Rationale and design of the TEMPO (tolvaptan efficacy and safety in management of autosomal dominant polycystic kidney disease and its outcomes) 3–4 study. Am J Kidney Dis. 2011;57(5):692–9.
Higashihara E, Torres VE, Chapman AB, et al. Tolvaptan in autosomal dominant polycystic kidney disease: three years’ experience. Clin J Am Soc Nephrol. 2011;6(10):2499–507.
Irazabal MV, Torres VE, Hogan MC, et al. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2011;80(3):295–301.
Boertien WE, Meijer E, de Jong PE, et al. Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am J Kidney Dis. 2015;65(6):833–41.
Boertien WE, Meijer E, de Jong PE, et al. Short-term renal hemodynamic effects of tolvaptan in subjects with autosomal dominant polycystic kidney disease at various stages of chronic kidney disease. Kidney Int. 2013;84(6):1278–86.
Shoaf SE, Bramer SL, Bricmont P, et al. Pharmacokinetic and pharmacodynamic interaction between tolvaptan, a non-peptide AVP antagonist, and furosemide or hydrochlorothiazide. J Cardiovasc Pharmacol. 2007;50(2):213–22.
Shoaf SE, Bricmont P, Mallikaarjun S. Pharmacokinetics and pharmacodynamics of oral tolvaptan in patients with varying degrees of renal function. Kidney Int. 2014;85(4):953–61.
Shoaf SE, Kim SR, Bricmont P, et al. Pharmacokinetics and pharmacodynamics of single-dose oral tolvaptan in fasted and non-fasted states in healthy Caucasian and Japanese male subjects. Eur J Clin Pharmacol. 2012;68(12):1595–603.
Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–18.
Torres VE, Devuyst O, Chapman AB, et al. Effect of tolvaptan in ADPKD by CKD stage: results from the TEMPO 3:4 trial [abstract plus poster]. In: World Congress of Nephrology; 2015.
Muto S, Kawano H, Higashihara E, et al. The effect of tolvaptan on autosomal dominant polycystic kidney disease patients: a subgroup analysis of the Japanese patient subset from TEMPO 3:4 trial. Clin Exp Nephrol. 2015. doi:10.1007/s10157-015-1086-2.
Devuyst O, Chapman AB, Boklage S, et al. Tolerability of aquaretic-related symptoms: results from the TEMPO 3:4 trial [abstract plus poster]. In: World Congress of Nephrology; 2015.
Watkins PB, Lewis JH, Kaplowitz N, et al. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of clinical trials database. Drug Saf. 2015. doi:10.1007/s40264-015-0327-3.
Nagao S, Nishii K, Katsuyama M, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol. 2006;17(8):2220–7.
Wang X, Wu Y, Ward CJ, et al. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19(1):102–8.
Torres VE, Wang X, Qian Q, et al. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med. 2004;10(4):363–4.
Ong AC, Devuyst O, Knebelmann B, et al. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet. 2015;385(9981):1993–2002.
Alam A, Dahl NK, Lipschutz JH, et al. Total kidney volume in autosomal dominant polycystic kidney disease: a biomarker of disease progression and therapeutic efficacy. Am J Kidney Dis. 2015. doi:10.1053/j.ajkd.2015.01.030.
Bhutani H, Smith V, Rahbari-Oskoui F, et al. A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int. 2015;88(1):146–51.
Otsuka Pharmaceutical Development and Commercialization. Open-label tolvaptan study in subjects with ADPKD (TEMPO 4/4). 2014. http://www.clinicaltrials.gov/ct2/show/NCT01214421. Accessed 18 Sep 2015.
Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan-treatment of ADPKD confers persistent EGFR improvement: results from the TEMPO 4:4 extension trial [abstract no. SO016]. In: 51st European Renal Association–European Dialysis and Transplant Association (ERA–EDTA) Congress; 2014.
Spital A. Tolvaptan in autosomal dominant polycystic kidney disease. N Engl J Med. 2013;368(13):1257.
Black P, Sutton R. Commentary on: Tolvaptan in patients with autosomal-dominant polycystic kidney disease. Urology. 2013;81(4):705–6.
Torres VE, Gansevoort RT, Czerwiec FS. Tolvaptan in autosomal dominant polycystic kidney disease. N Engl J Med. 2013;368(13):1259.
National Institute for Health and Care Excellence. Final appraisal determination document—tolvaptan for treating autosomal dominant polycystic kidney disease. 2015. http://www.nice.org.uk. Accessed 18 Sep 2015.
Robinson P, McEwan P, Ong ACM, et al. Assessing the long term outcomes of autosomal dominant polycystic kidney disease (ADPKD) using the ADPKD outcomes model: a UK case study [abstract no. FP064 plus poster]. In: 52nd European Renal Association–European Dialysis and Transplant Association (ERA–EDTA) Congress. 2015.
O’Reilly K, McEwan P, Bennett Wilton H, et al. Exploring the impact of tolvaptan on the long term rate of renal function decline using the ADPKD outcomes model [abstract no. FP055 plus poster]. In: 52nd European Renal Association–European Dialysis and Transplant Association (ERA–EDTA) Congress. 2015.
Blanchette CM, Matter S, Chawla A, et al. Burden of autosomal dominant polycystic kidney disease: systemic literature review. Am J Pharm Benefits. 2015;7(2):e27–36.
Otsuka Pharmaceutical Development and Commercialization. Efficacy and safety of tolvaptan in subjects with chronic kidney disease between late stage 2 to early stage 4 due to autosomal dominant polycystic kidney disease. 2014. http://www.clinicaltrials.gov/ct2/show/NCT02160145. Accessed 18 Sep 2015.
Otsuka Pharmaceutical Development and Commercialization. Open-label trial to evaluate the long term safety of titrated immediate-release tolvaptan in subjects with autosomal dominant polycystic kidney disease. 2014. http://www.clinicaltrials.gov/ct2/show/NCT02251275. Accessed 18 Sep 2015.
Acknowledgments
During the peer review process, the manufacturer of tolvaptan was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
Hannah Blair and Gillian Keating are salaried employees of Adis/Springer, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: D. O’Donoghue, Salford Royal NHS Foundation Trust, Salford, UK; R. Torra, Inherited Kidney Diseases, Nephrology Department, Fundacio Puigvert, Barcelona, Spain; C. Willey, College of Pharmacy, University of Rhode Island, Kingston, RI, USA.
Rights and permissions
About this article
Cite this article
Blair, H.A., Keating, G.M. Tolvaptan: A Review in Autosomal Dominant Polycystic Kidney Disease. Drugs 75, 1797–1806 (2015). https://doi.org/10.1007/s40265-015-0475-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0475-x