Abstract
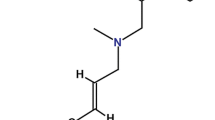
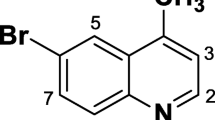
Tavaborole is a novel, low-molecular weight oxaborole antifungal drug under development by Anacor Pharmaceuticals Inc. for the topical treatment of onychomycosis of the toenail. The drug has received its first global approval for this indication in the US. This article summarizes the milestones in the development of tavaborole leading to this first approval for onychomycosis of the toenails.
Similar content being viewed by others
References
Elewski B, Pariser D, Rich P, et al. Current and emerging options in the treatment of onychomycosis. Semin Cutan Med Surg. 2013;32(2 Suppl 1):S9–12.
Anacor Pharmaceuticals Inc. FDA approves Anacor Pharmaceuticals KERYDINTM (tavaborole) topical solution, 5 % for the treatment of onychomycosis of the toenails [media release]. 2014. http://investor.anacor.com/releasedetail.cfm?ReleaseID=858211.
Anacor Pharmaceuticals Inc. Tavaborole KERYDINTM (tavaborole): US prescribing information. 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204427s000lbl.pdf. Accessed 24 Jul 2014.
Anacor Pharmaceuticals Inc. Anacor regains worldwide rights to AN2690 from Merck. [media release]. 2010. http://investor.anacor.com/releasedetail.cfm?ReleaseID=527619.
Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316(5832):1759–61.
Sanders V, Baker S, Alley M. Microbiological activity of AN2690, a new antifungal agent in development for the topical treatment of onychomycosis [poster]. 2006. http://www.anacor.com/pdf/AAD_P1608.pdf. Accessed 6 Aug 2014.
Hui X, Baker SJ, Wester RC, et al. In vitro penetration of a novel oxaborole antifungal (AN2690) into the human nail plate. J Pharm Sci. 2007;96(10):2622–31.
Elewski BE, Tosti A. Tavaborole for the treatment of onychomycosis. Expert Opin Pharmacother. 2014;15(10):1439–48.
Beutner K, Sanders V, Hold K. An open-label, multiple-dose study of the absorption and systemic pharmacokinetics of AN2690 applied as a 7.5 % solution to all toenails of adult patients with moderate to severe onychomycosis. J Am Acad Dermatol. 2007;56(2 Suppl 2):129.
Dosik J, Lastella P, Yavel R, et al. The pharmacokinetics of AN2690 after topical application in patients with onychomycosis. J Invest Dermatol. 2011;131:S47.
Elewski BE, Rich P, Wiltz H, et al. Effectiveness and safety of tavaborole, a novel boron-based molecule for the treatment of onychomycosis: results from two phase 3 studies [poster]. In: 10th Annual High Risk Diabetic Foot Conference, Phoenix, 20–22 Nov 2013.
Toledo Bahena M, Gomez JB, Zane L, et al. Safety and efficacy of tavaborole (AN2690), a novel boron-based molecule, in 3 phase II trials for the topical treatment of toenail onychomycosis. J Am Acad Dermatol. 2014;70(5 Suppl 1):AB92.
Zane L, Pollak R. Safety and efficacy of tavaborole (formerly AN2690), a novel boron-based molecule, in phase 2 trials for the topical treatment of toenail onychomycosis [poster no. P1608]. In: 100th National American Podiatric Medical Association, Washington DC, 16–19 August 2012.
Disclosure
The preparation of this report was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. A. Markham is a contracted employee of Adis, Springer SBM.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the Adis R&D Insight drug pipeline database. Adis R&D Insight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch.
Rights and permissions
About this article
Cite this article
Markham, A. Tavaborole: First Global Approval. Drugs 74, 1555–1558 (2014). https://doi.org/10.1007/s40265-014-0276-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0276-7