Abstract
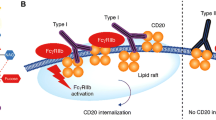
Obinutuzumab (Gazyva™) is an intravenously administered, humanized and glycoengineered, type II anti-CD20 monoclonal antibody for the treatment of B-cell malignancies. It is approved in the US for use in combination with chlorambucil for the first-line treatment of chronic lymphocytic leukaemia (CLL), and has been filed for approval in the EU in this indication. The antibody is based on GlycArt Biotechnology’s (later Roche Glycart AG) proprietary GlycoMAb® technology, which uses glycoengineered antibodies that specifically increase antibody-dependent cellular cytotoxicity and thereby increase immune-mediated target cell death. Obinutuzumab is a type II anti-CD20 antibody that induces enhanced direct cell death. The monoclonal antibody is in worldwide phase III development with Roche and its subsidiaries, Genentech and Chugai Pharmaceutical, as well as Biogen Idec, for diffuse large B-cell lymphoma and non-Hodgkin’s lymphoma generally, and is also in phase III development in countries outside of the US and EU for CLL.
Similar content being viewed by others
References
Seiler TM, Hiddemann W. Advances in the management of follicular lymphoma. Curr Opin Oncol. 2012;24(6):742–7.
Goede V, Hallek M. Optimal pharmacotherapeutic management of chronic lymphocytic leukaemia: considerations in the elderly. Drugs Aging. 2011;28:163–76.
Chang JE, Kahl BS. Current status of targeted therapies for mantle cell lymphoma. Drugs. 2011;71:2307–26.
Merli M, Ferrario A, Basilico C, et al. Novel agents in indolent lymphomas. Ther Adv Hematol. 2013;4(2):133–48.
Klein C, Lammens A, Schafer W, et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. mAbs. 2013;5(1):22–33.
Honeychurch J, Alduaij W, Azizyan M, et al. Antibody-induced nonapoptotic cell death in human lymphoma and leukemia cells is mediated through a novel reactive oxygen species-dependent pathway. Blood. 2012;119(15):3523–33.
US FDA. FDA approves Gazyva for chronic lymphocytic leukemia [media release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm373209.htm. Accessed 1 Nov 2013.
US FDA. Gazyva (obinutuzumab): US prescribing information. 2013. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125486s000lbl.pdf. Accessed 14 Nov 2013.
Moritoki YB, Lian ZX, Lindor K, et al. Cell depletion with anti-CD20 ameliorates autoimmune cholangitis but exacerbates colitis in transforming growth factor-beta receptor II dominant negative mice. Hepatology. 2009;50(6):1893–903.
Chugai Pharmaceutical Co Ltd, Nippon Shinyaku Co Ltd. Co-development and co-marketing agreement for the glycoengineered type II anti-CD20 monoclonal antibody, “GA101 (obinutuzumab)” [media release]. http://www.chugai-pharm.co.jp. Accessed 27 Nov 2012.
Genentech, Roche. Genentech, GlycArt and Roche enter into collaboration agreement for potential cancer therapy [media release]. http://www.gene.com. Accessed 3 Oct 2008.
Biogen Idec. Biogen Idec elects to participate with Genentech in the development and commercialization of a next generation anti-CD20 molecule [media release]. http://www.biogenidec.com. Accessed 31 Oct 2008.
Biogen Idec, Genentech Inc. Biogen Idec and Genentech announce restructuring of anti-CD20 collaboration agreement [media release]. http://www.gene.com. Accessed 22 Oct 2010.
GlycArt Biotechnology AG. Acquisition of GlycArt Biotechnology completed [media release]. http://www.glycart.com. Accessed 26 Jul 2005.
Niederfellner G, Lammens A, Mundigl O, et al. Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/II distinction of CD20 antibodies. Blood. 2011;118(2):358–67.
Mossner E, Brunker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–402.
Herter S, Herting F, Mundigl O, et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther. 2013;12(10):2031–42.
Rafiq S, Butchar JP, Cheney C, et al. Comparative assessment of clinically utilized CD20-directed antibodies in chronic lymphocytic leukemia cells reveals divergent NK cell, monocyte, and macrophage properties. J Immunol. 2013;190(6):2702–11.
Cartron G, Morschhauser F, Thieblemont C, et al. Results from a phase II study of obinutuzumab (GA101) monotherapy in relapsed/refractory chronic lymphocytic leukemia (CLL) [abstract no. 0101]. Haematologica. 2011;96(Suppl 2):39–40.
Morschhauser F, Cartron G, Lamy T, et al. Phase I study of RO5072759 (GA101) in relapsed/refractory chronic lymphocytic leukemia [abstract no. 884]. Blood. 2009;114 Suppl.
Salles G, Morschhauser F, Lamy T, et al. Phase I study of RO5072759 (GA101) in patients with relapsed/refractory CD20+ non-Hodgkin lymphoma [abstract no. 1704]. Blood. 2009;114 Suppl.
Sehn LH, Assouline SE, Stewart DA, et al. A phase I study of GA101 (RO5072759) monotherapy followed by maintenance in patients with multiply relapsed/refractory CD20+ malignant disease [abstract no. 934]. Blood. 2009;114 Suppl.
Meneses-Lorente G, Carlile D, Birkett J, et al. Pharmacokinetics of RO5072759 (GA101) in patients with relapsed/refractory CD20+ malignant disease (phase I/II study BO20999) [abstract no. 1833]. Blood. 2010;116.
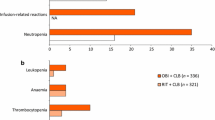
Goede V, Fischer K, Busch R, et al. Head-to-head comparison of obinutuzumab (GA101) plus chlorambucil (Clb) versus rituximab plus Clb in patients with chronic lymphocytic leukemia (CLL) and co-existing medical conditions (comorbidities): final stage 2 results of the CLL11 trial. 55th American Society of Hematology Annual Meeting and Exposition, 7–10 Dec, New Orleans. 2013. https://ash.confex.com/ash/2013/webprogram/Paper60276.html.
Hallek M, Fischer K, Humphrey K, et al. Obinutuzumab (GA101) + chlorambucil (CLB) or rituximab (r) + Clb versus Clb alone in patients with chronic lymphocytic leukaemia (CLL) and pre-existing medical conditions (comorbidities): final stage 1 results of the CLL11 (BO21004) phase 3 trial [abstract no. 056]. Hematol Oncol. 2013;31(Suppl 1):114–5.
Goy A, Offner F, Martinelli G, et al. Randomized phase II trial comparing obinutuzumab (GA101) with rituximab in patients with relapsed CD20+ indolent B-cell non-Hodgkin lymphoma: preliminary analysis of the GAUSS study [abstract no. 0790]. Haematologica. 2012;97(Suppl 1):322–33.
Morschhauser FA, Cartron G, Thieblemont C, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31(23):2912–9.
Salles GA, Morschhauser F, Solal-Celigny P, et al. Obinutuzumab (GA101) in patients with relapsed/refractory indolent non-Hodgkin lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31(23):2920–6.
Salles G, Morschhauser F, Lamy T, et al. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012;119(22):5126–32.
Dyer MJS, Grigg A, Gonzalez M, et al. Obinutuzumab (GA101) in combination with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) or bendamustine in patients with previously untreated follicular lymphoma (FL): results of the phase Ib GAUDI study (BO21000) [abstract no. 3686]. Blood. 2012;120(21).
Radford J, Davies A, Cartron G, et al. Obinutuzumab (GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma: results of the GAUDI study (BO21000). Blood. 2013;122(7):1137–43.
Brown JR, O’Brien S, Kingsley CD, et al. Safety and efficacy of obinutuzumab (GA101) with fludarabine/cyclophosphamide (G-FC) or bendamustine (G-B) in the initial therapy of patients with chronic lymphocytic leukemia (CLL): results from the phase 1b Galton trial (GAO4779g). 55th American Society of Hematology Annual Meeting and Exposition, 7–10 Dec, New Orleans. 2013. https://ash.confex.com/ash/2013/webprogram/Paper63449.html.
Sehn LH, Assouline SE, Stewart DA, et al. A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood. 2012;119(22):5118–25.
Ogura M, Tobinai K, Hatake K, et al. Phase I study of obinutuzumab (GA101) in Japanese patients with relapsed or refractory B-cell non-Hodgkin lymphoma. Cancer Sci. 2013;104(1):105–10.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the Adis R&D Insight drug pipeline database. Adis R&D Insight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch.
Rights and permissions
About this article
Cite this article
Cameron, F., McCormack, P.L. Obinutuzumab: First Global Approval. Drugs 74, 147–154 (2014). https://doi.org/10.1007/s40265-013-0167-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-013-0167-3