Abstract
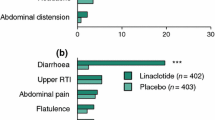
Linaclotide (Constella®) is a synthetic 14-amino acid peptide, structurally related to guanylin and uroguanylin, that acts as a potent guanylate cyclase C receptor agonist. It is a first-in-class agent recently approved in the EU for the treatment of adult patients with moderate to severe irritable bowel syndrome with constipation (IBS-C). Linaclotide has very low oral bioavailability and acts locally in the gastrointestinal tract to stimulate fluid secretion, increase colonic transit, and reduce abdominal pain. In phase III trials, once-daily, oral linaclotide significantly increased compared with placebo the proportions of 12-week abdominal pain/discomfort responders and 12-week degree-of-relief responders (co-primary endpoints recommended by the European Medicines Agency). Linaclotide also significantly increased the proportions of responders at 26 weeks compared with placebo, and significantly improved all abdominal symptoms and measures of bowel function at 12 weeks compared with placebo. In addition, linaclotide generally improved health-related quality of life compared with placebo. Linaclotide was generally well tolerated; the most common adverse event was diarrhoea. Thus, linaclotide is a novel and effective single agent for the treatment of IBS-C in adults.

Similar content being viewed by others
References
Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91.
Suares NC, Ford AC. Diagnosis and treatment of irritable bowel syndrome. Discov Med. 2011;11(60):425–33.
Hungin AP, Chang L, Locke GR, et al. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther. 2005;21(11):1365–75.
Hungin AP, Whorwell PJ, Tack J, et al. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17(5):643–50.
European Medicines Agency. Constella (linaclotide): summary of product characteristics. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002490/WC500135622.pdf. Accessed 7 Nov 2013.
US FDA. Linzess (linaclotide) capsules: US prescribing information. 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202811s000lbl.pdf. Accessed 7 Nov 2013.
Busby RW, Kessler MM, Bartolini WP, et al. Pharmacologic properties, metabolism, and disposition of linaclotide, a novel therapeutic peptide approved for the treatment of irritable bowel syndrome with constipation and chronic idiopathic constipation. J Pharmacol Exp Ther. 2013;344(1):196–206.
Fonteles MC, do Nascimento NRF. Guanylin peptide family: history, interactions with ANP, and new pharmacological perspectives. Can J Physiol Pharmacol. 2011;89(8):575–85.
Forte LR Jr. Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics. Pharmacol Ther. 2004;104(2):137–62.
Bryant AP, Busby RW, Bartolini WP, et al. Linaclotide is a potent and selective guanylate cyclase C agonist that elicits pharmacological effects locally in the gastrointestinal tract. Life Sci. 2010;86(19–20):760–5.
Busby RW, Bryant AP, Bartolini WP, et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol. 2010;649(1–3):328–35.
Andresen V, Camilleri M, Busciglio IA, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007;133(3):761–8.
Eutamene H, Bradesi S, Larauche M, et al. Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain. Neurogastroenterol Motil. 2010;22(3):312–e84.
Silos-Santiago I, Hannig G, Eutamene H, et al. Gastrointestinal pain: unravelling a novel endogenous pathway through uroguanylin/guanylate cyclase-C/cGMP activation. Pain. 2013;154(9):1820–30.
Castro J, Harrington AM, Hughes PA, et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic GMP. Gastroenterology. 2013. doi:10.1053/j.gastro.2013.08.017.
Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107(11):1702–12.
Rao S, Lembo AJ, Shiff SJ, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012;107(11):1714–24.
US FDA. Guidance for industry: irritable bowel syndrome - clinical evaluation of drugs for treatment. 2012. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM205269.pdf. Accessed 7 Nov 2013.
Quigley EMM, Tack J, Chey WD, et al. Randomised clinical trials: linaclotide phase 3 studies in IBS-C: a prespecified further analysis based on European Medicines Agency-specified endpoints. Aliment Pharmacol Ther. 2013;37(1):49–61.
European Medicines Agency. Points to consider on the evaluation of medicinal products for the treatment of irritable bowel syndrome. 2003. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003187.pdf. Accessed 7 Nov 2013.
Johnston JM, Kurtz CB, MacDougall JE, et al. Linaclotide improves abdominal pain and bowel habits in a phase IIb study of patients with irritable bowel syndrome with constipation. Gastroenterology. 2010;139(6):1877–86.e2.
Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl II):II43–7.
Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920–4.
Chang L, Lembo A, Shiff S, et al. Effects of linaclotide on abdominal and bowel symptoms over the first seven days of treatment in patients with irritable bowel syndrome (IBS) with constipation [abstract no. 214]. Neurogastroenterol Motil. 2012;24(Suppl s2):105.
Chang L, Lembo A, Shiff S, et al. Effects of linaclotide on abdominal and bowel symptoms over the first seven days of treatment in patients with irritable bowel syndrome with constipation [abstract no. 1747]. Am J Gastroenterol. 2012;107(Suppl 1):S710–1.
Buono JL, Tourkodimitris S, Sarocco P, et al. Impact of linaclotide treatment on work productivity and activity impairment in adults with irritable bowel syndrome with constipation. Value Health. 2012;15(7):A330–1.
National Institute for Health and Clinical Excellence. Irritable bowel syndrome in adults: diagnosis and management of irritable bowel syndrome in primary care (NICE clinical guideline 61). 2008. http://www.nice.org.uk/nicemedia/pdf/CG061NICEGuideline.pdf. Accessed 7 Nov 2013.
World Gastroenterology Organisation. World Gastroenterology Organisation global guideline: irritable bowel syndrome: a global perspective. 2009. http://www.guideline.gov/content.aspx?id=15232&search=irritable+bowel. Accessed 7 Nov 2013.
US FDA. Amitiza (lubiprostone) capsules: US prescribing information. 2013. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021908s011lbl.pdf. Accessed 7 Nov 2013.
US FDA. Zelnorm (tegaserod maleate) information. 2012. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm103223.htm. Accessed 7 Nov 2013.
Pitari GM. Pharmacology and clinical potential of guanylyl cyclase C agonists in the treatment of ulcerative colitis. Drug Des Dev Ther. 2013;7:351–60.
Huang H, Taylor DCA, Carson RT, et al. Cost-effectiveness of linaclotide for the treatment of adult patients in the US with irritable bowel syndrome with constipation [abstract no. PG112]. Value Health. 2013;16(3):A213.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: M. Camilleri, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic College of Medicine, Rochester, MN, USA; E.M.M. Quigley, Alimentary Pharmabiotic Centre, University College Cork, Cork, Ireland.
Rights and permissions
About this article
Cite this article
McCormack, P.L. Linaclotide: A Review of Its Use in the Treatment of Irritable Bowel Syndrome with Constipation. Drugs 74, 53–60 (2014). https://doi.org/10.1007/s40265-013-0157-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-013-0157-5