Abstract
Background and Objective
Over the past 2 years, several drugs have been approved for coronavirus disease 2019 (COVID-19) treatment, but their safety during pregnancy remains poorly understood. This study aims to assess the relative risk of obstetric, neonatal, and infant outcomes associated with the use of drugs specifically indicated for the treatment of COVID-19 compared with other drug treatment strategies. The purpose of this article is to present elements of the study protocol.
Methods
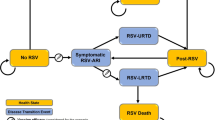
The COVID-19 International Drug Pregnancy Registry (COVID-PR) is a noninterventional, postmarketing cohort study. Pregnant women receiving treatment with monoclonal antibodies (mAbs) or antiviral drugs for mild, moderate, or severe COVID-19 are matched 1:1 with pregnant women not receiving these study-specific drugs, based on calendar time, country, gestational age at enrollment, and COVID-19 severity. Participants complete online questionnaires at enrollment, during pregnancy, and for 12 months after delivery of liveborn infants. The study began enrolling participants on 1 December 2021 and is set to span 5 years for each drug of interest.
Discussion
The COVID-PR is designed to evaluate the safety profile of each studied drug. Additionally, it may allow for an analysis of the effects of COVID-19 drug exposure during relevant gestational periods on specific neonatal outcomes. Although the sample size will be too small to detect associations with rare outcomes, the study has the potential to generate hypotheses for future research. Ultimately, these data can provide valuable insights for evidence-based decisions about COVID-19 treatment during pregnancy.
Trial registration: ClinicalTrials.gov: NCT05013632. EU PAS EUPAS42517.

Similar content being viewed by others
References
Adam S, Pheiffer C, Dias S, Hlongwane T, Vannevel V, Soma-Pillay P, et al. Coronavirus and pregnancy: the challenges of the 21(st) century: a review. Front Microbiol. 2022;13: 923546.
Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy. Am J Obstet Gynecol. 2022;226(2):177–86.
Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021;175(8):817–26.
Villar J, Soto CCP, Gunier RB, Ariff S, Craik R, Cavoretto PI, et al. Pregnancy outcomes and vaccine effectiveness during the period of omicron as the variant of concern, INTERCOVID-2022: a multinational, observational study. Lancet. 2023;401(10375):447–57.
Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):769–75.
Collin J, Bystrom E, Carnahan A, Ahrne M. Public Health Agency of Sweden’s brief report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99(7):819–22.
Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370: m3320.
Male V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat Rev Immunol. 2022;22(5):277–82.
Regulatory Affairs Professionals Society. COVID-19 therapeutics tracker 2022. Available from https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-therapeutics-tracker. Accessed August 16, 2022.
Pidiyar V, Kumraj G, Ahmed K, Ahmed S, Shah S, Majumder P, et al. COVID-19 management landscape: a need for an affordable platform to manufacture safe and efficacious biotherapeutics and prophylactics for the developing countries. Vaccine. 2022;40:5302–12.
World Health Organization (WHO). Therapeutics and COVID-19: living guideline 2023 [v13.1]. Available from: https://app.magicapp.org/#/guideline/nBkO1E. Accessed April 5, 2023.
U.S. National Institutes of Health. Covid-19 treatment guidelines: clinical management of adults 2023. [updated May 2, 2023]. Available from https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/clinical-management-of-adults-summary/. Accessed June 19, 2023.
Kumari M, Lu RM, Li MC, Huang JL, Hsu FF, Ko SH, et al. A critical overview of current progress for COVID-19: development of vaccines, antiviral drugs, and therapeutic antibodies. J Biomed Sci. 2022;29(1):68.
U.S. Food and Drug Administration. Fact sheet for healthcare providers: Emergency use authorization for Lagevrio (molnupiravir) capsules 2022. Available from https://www.fda.gov/media/155054/download. Accessed: August 17, 2022.
Altafim ERP, McCoy DC, Brentani A, Escobar AMU, Grisi S, Fink G. Measuring early childhood development in Brazil: validation of the caregiver reported early development instruments (CREDI). J Pediatr (Rio J). 2020;96(1):66–75.
Li Y, Tang L, Bai Y, Zhao S, Shi Y. Reliability and validity of the caregiver reported early development instruments (CREDI) in impoverished regions of China. BMC Pediatr. 2020;20(1):475.
U.S. Centers for Disease Control and Prevention. Birth defects research and tracking 2021 [updated July 21, 2021]. Available from https://www.cdc.gov/ncbddd/birthdefects/data.html. Accessed August 9, 2022.
U.S. Food and Drug Administration. 2023. Available from https://www.fda.gov. Accessed June 19, 2023.
European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP). 2023. Available from: https://www.encepp.eu. Accessed June 19, 2023.
U.S. National Library of Medicine. ClinicalTrials.gov 2023. Available from: https://clinicaltrials.gov/. Accessed June 19, 2023.
Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30.
European Commission. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation). OJ2016. p. 1–88.
International Committee of Medical Journal Editors. Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals 2019. Available from: http://www.icmje.org/recommendations/. Accessed July 27, 2022.
Margulis AV, Mittleman MA, Glynn RJ, Holmes LB, Hernandez-Diaz S. Effects of gestational age at enrollment in pregnancy exposure registries. Pharmacoepidemiol Drug Saf. 2015;24(4):343–52.
McCoy DC, Sudfeld CR, Bellinger DC, Muhihi A, Ashery G, Weary TE, et al. Development and validation of an early childhood development scale for use in low-resourced settings. Popul Health Metr. 2017;15(1):3.
Acknowledgements
Editorial assistance for this manuscript was provided by Alison Thornton (freelance medical writer, funded by Pregistry), Helen Dolk, and Mondira Bhattacharya.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The COVID-PR receives funding from the following sources: GSK, Merck Sharp and Dohme, Roche-Regeneron, and Gilead.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
The study protocol has been approved in the USA by the WCG IRB (20214420), in Japan by Medical Corporation TOUKEIKAI Kitamachi Clinic ERB (RVG0947720230315), and in Australia by The Bellberry Human Research Ethics Committee (2022-04-403). Clearance that approval is not required to conduct the study has been given by Ethics Committees in the following countries: Bulgaria, Canada, Croatia, Czech Republic, Denmark, Germany, Greece, Hong Kong, Italy, Malaysia, New Zealand, Poland, Romania, Singapore, South Africa, South Korea, and the UK. The study will be performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to Participate
Participants will provide informed written consent before taking part in the study.
Consent for Publication
Participants will provide informed written consent before taking part in the study.
Availability of Data and Materials
The COVID-PR protocol may be made available by the corresponding author on reasonable request, following applicable regulations.
Code Availability
Not applicable.
Author Contributions
The original study protocol was designed by DFW and SH-D. DFW and CR supervised data collection and data verification. SH-D, CR, ATP, and TDM provided advice on the study methodology and edited the first and final versions of the article. All authors have read and approved the final version of the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wyszynski, D.F., Papageorghiou, A.T., Renz, C. et al. The COVID-19 International Drug Pregnancy Registry (COVID-PR): Protocol Considerations. Drug Saf 47, 195–204 (2024). https://doi.org/10.1007/s40264-023-01377-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01377-2