Abstract
Background and Objective
Prior molecular modelling analysis identified several medicines as potential inhibitors of glutathione peroxidase 1 (GPx1) which may contribute to development or progression of chronic obstructive pulmonary disease (COPD). This study investigates 40 medicines (index medicines) for signals of COPD development or progression in a real-world dataset.
Methods
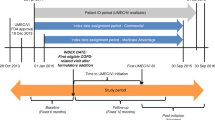
Sequence symmetry analysis (SSA) was conducted using a 10% extract of Australian Pharmaceutical Benefits Scheme (PBS) claims data between January 2013 and September 2019. Patients must have been initiated on an index medicine and a medicine for COPD development or progression within 12 months of each other. Sequence ratios were calculated as the number of patients who initiated an index medicine followed by a medicine for COPD development or progression divided by the number who initiated the index medicine second. An adjusted sequence ratio (aSR) was calculated which accounted for changes in prescribing trends. Adverse drug event signals (ADEs) were identified where the aSR lower 95% confidence interval (CI) was greater than 1.
Results
Twenty-one of 40 (53%) index medicines had at least one ADE signal of COPD development or progression. Signals of COPD development, as identified using initiation of tiotropium, were observed for atenolol (aSR 1.32, 95% CI 1.23–1.42) and naproxen (aSR 1.14, 95% CI 1.06–1.23). Several signals of COPD progression were observed, including initiation of fluticasone propionate/salmeterol following initiation of atenolol (aSR 1.44, 95% CI 1.30–1.60) and initiation of aclidinium/formoterol following initiation of naproxen (aSR 2.21, 95% CI 1.34–3.65).
Conclusion
ADE signals were generated for several potential GPx1 inhibitors; however, further validation of signals is required in large well-controlled observational studies.





Similar content being viewed by others
References
Lung Foundation Australia. Overview chronic obstructive pulmonary disease. 2022 [cited 16 February 2022]. Available from: https://lungfoundation.com.au/patients-carers/living-with-a-lung-disease/copd/overview/.
Australian Institute of Health and Welfare. Chronic obstructive pulmonary disease (COPD). [Cited16 February 2022]. Available from: https://www.aihw.gov.au/reports/chronic-respiratory-conditions/copd/contents/copd.
Blanco I, Diego I, Bueno P, Casas-Maldonado F, Miravitlles M. Geographic distribution of COPD prevalence in the world displayed by Geographic Information System maps. Eur Respir J. 2019;54(1):1900610.
World Health Organization. Chronic obstructive pulmonary disease (COPD)
Diez-Manglano J, Barquero-Romero J, Mena PA, Recio-Iglesias J, Cabrera-Aguilar J, Lopez-Garcia F, et al. Polypharmacy in patients hospitalised for acute exacerbation of COPD. Eur Respir J. 2014;44(3):791–4.
Boom M, Niesters M, Sarton E, Aarts L, Smith TW, Dahan A. Non-analgesic effects of opioids: opioid-induced respiratory depression. Curr Pharm Des. 2012;18(37):5994–6004.
Prosser AE, Dawson JL, Koo K, O’Kane KM, Ward MB, Woodman RJ, et al. Real-world incidence of patient-reported dyspnoea with ticagrelor. Ther Adv Drug Saf. 2018;9(10):577–84.
Miller KL, Sawitzke AD, Doane J. Abatacept and serious respiratory infections in patients with previous lung disease. Clin Rheumatol. 2008;27(12):1569–71.
Australian Medicines H. Australian medicines handbook: AMH. AMH; 2022.
Garcia Rodriguez LA, Wallander MA, Tolosa LB, Johansson S. Chronic obstructive pulmonary disease in UK primary care: incidence and risk factors. COPD. 2009;6(5):369–79.
Jacobson GA, Yee KC, Ng CH. Elevated plasma glutathione peroxidase concentration in acute severe asthma: comparison with plasma glutathione peroxidase activity, selenium and malondialdehyde. Scand J Clin Lab Investig. 2007;67(4):423–30.
Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol. 2003;111(1):72–8.
Vlahos R, Bozinovski S. Glutathione peroxidase-1 as a novel therapeutic target for COPD. Redox Rep. 2013;18(4):142–9.
Rahman I. Pharmacological antioxidant strategies as therapeutic interventions for COPD. Biochim Biophys Acta. 2012;1822(5):714–28.
Calzetta L, Rogliani P, Facciolo F, Rinaldi B, Cazzola M, Matera MG. N-Acetylcysteine protects human bronchi by modulating the release of neurokinin A in an ex vivo model of COPD exacerbation. Biomed Pharmacother. 2018;103:1–8.
Zinellu E, Zinellu A, Pau MC, Piras B, Fois AG, Mellino S, et al. Glutathione peroxidase in stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. Antioxidants (Basel). 2021;10(11):1745.
Janetzki JL, Pratt NL, Ward MB, Sykes MJ. Application of an integrative drug safety model for detection of adverse drug events associated with inhibition of glutathione peroxidase 1 in chronic obstructive pulmonary disease. Pharm Res. 2023;40:1553–68.
Lai EC, Pratt N, Hsieh CY, Lin SJ, Pottegard A, Roughead EE, et al. Sequence symmetry analysis in pharmacovigilance and pharmacoepidemiologic studies. Eur J Epidemiol. 2017;32(7):567–82.
Pratt NL, Ilomaki J, Raymond C, Roughead EE. The performance of sequence symmetry analysis as a tool for post-market surveillance of newly marketed medicines: a simulation study. BMC Med Res Methodol. 2014;14(1):66.
King CE, Pratt NL, Craig N, Thai L, Wilson M, Nandapalan N, et al. Detecting medicine safety signals using prescription sequence symmetry analysis of a national prescribing data set. Drug Saf. 2020;43(8):787–95.
Roughead EE, Chan EW, Choi NK, Griffiths J, Jin XM, Lee J, et al. Proton pump inhibitors and risk of Clostridium difficile infection: a multi-country study using sequence symmetry analysis. Expert Opin Drug Saf. 2016;15(12):1589–95.
World Health Organization Collaborating Centre for Drug Statistics Methodology. Anatomical therapeutic chemical code classification index with defined daily doses.
Australian Government Department of Health. Pharmaceutical benefits scheme. 2022 [cited 7 April 2022]. Available from: https://www.pbs.gov.au/pbs/home.
Toelle BG, Xuan W, Bird TE, Abramson MJ, Atkinson DN, Burton DL, et al. Respiratory symptoms and illness in older Australians: the Burden of Obstructive Lung Disease (BOLD) study. Med J Aust. 2013;198(3):144–8.
Liu Y, Pleasants RA, Croft JB, Wheaton AG, Heidari K, Malarcher AM, et al. Smoking duration, respiratory symptoms, and COPD in adults aged >/=45 years with a smoking history. Int J Chron Obstr Pulm Dis. 2015;10:1409–16.
Janetzki JL, Sykes MJ, Ward MB, Pratt NL. Proton pump inhibitors may contribute to progression or development of chronic obstructive pulmonary disease—a sequence symmetry analysis approach. J Clin Pharm Ther. 2021;46(6):1687–94.
Lung Foundation Australia. Stepwise Management of Stable COPD. 2020.
Preiss AK, Roughead EE, Pratt NL. Sequence symmetry analysis graphic adjustment for prescribing trends. BMC Med Res Methodol. 2019;19(1):143.
Baker JG, Wilcox RG. Beta-Blockers, heart disease and COPD: current controversies and uncertainties. Thorax. 2017;72(3):271–6.
MIMS Australia. MIMS Online. Monthly index of medical specialities. St Leonards, N.S.W.: MIMS Australia; 1996.
Gulea C, Zakeri R, Alderman V, Morgan A, Ross J, Quint JK. Beta-blocker therapy in patients with COPD: a systematic literature review and meta-analysis with multiple treatment comparison. Respir Res. 2021;22(1):64.
Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125(6):2309–21.
Dorow P, Thalhofer S, Bethge H, Disselhoff G, Wagner G. Long-term treatment of angina pectoris with bisoprolol or atenolol in patients with chronic obstructive bronchitis: a randomized, double-blind crossover study. J Cardiovasc Pharmacol. 1990;16(Suppl 5):S36-44.
van Zyl AI, Jennings AA, Bateman ED, Opie LH. Comparison of respiratory effects of two cardioselective beta-blockers, celiprolol and atenolol, in asthmatics with mild to moderate hypertension. Chest. 1989;95(1):209–13.
Voiriot G, Philippot Q, Elabbadi A, Elbim C, Chalumeau M, Fartoukh M. Risks related to the use of non-steroidal anti-inflammatory drugs in community-acquired pneumonia in adult and pediatric patients. J Clin Med. 2019;8(6):786.
Orhan H, Sahin G. In vitro effects of NSAIDS and paracetamol on oxidative stress-related parameters of human erythrocytes. Exp Toxicol Pathol. 2001;53(2–3):133–40.
McKeever TM, Lewis SA, Smit HA, Burney P, Britton JR, Cassano PA. The association of acetaminophen, aspirin, and ibuprofen with respiratory disease and lung function. Am J Respir Crit Care Med. 2005;171(9):966–71.
Montuschi P, Macagno F, Parente P, Valente S, Lauriola L, Ciappi G, et al. Effects of cyclo-oxygenase inhibition on exhaled eicosanoids in patients with COPD. Thorax. 2005;60(10):827–33.
Health AIo, Welfare. Chronic obstructive pulmonary disease (COPD), associated comorbidities and risk factors. Canberra: AIHW; 2020.
Therapeutic Guidelines. Therapeutic Guidelines. eTG complete. West Melbourne: Therapeutic Guidelines Ltd.; 2003.
Geraghty P, Baumlin N, Salathe MA, Foronjy RF, D’Armiento JM. Glutathione peroxidase-1 suppresses the unfolded protein response upon cigarette smoke exposure. Mediat Inflamm. 2016;2016:9461289.
Sethi S. Infection as a comorbidity of COPD. Eur Respir J. 2010;35(6):1209–15.
Dalmarco EM, Budni P, Parisotto EB, Wilhelm Filho D, Frode TS. Antioxidant effects of mycophenolate mofetil in a murine pleurisy model. Transpl Immunol. 2009;22(1–2):12–7.
Faillie JL. Case-non-case studies: principle, methods, bias and interpretation. Therapie. 2019;74(2):225–32.
Hersom K, Neary MP, Levaux HP, Klaskala W, Strauss JS. Isotretinoin and antidepressant pharmacotherapy: a prescription sequence symmetry analysis. J Am Acad Dermatol. 2003;49(3):424–32.
Pharmaceutical Benefits Scheme. Post-market review of chronic obstructive pulmonary disease medicines background and tor 1 final report. 2017.
Australian Government Department of Health. PBS publications archive: schedule of pharmaceutical benefits (Summary of Changes) 1 June 2021. 2022 [cited 24 February 2022]. Available from: https://www.pbs.gov.au/publication/schedule/2018/06/2018-06-01-general-soc.pdf.
Hallas J, Whitaker H, Delaney JA, Cadarette SM, Pratt N, Maclure M. The use of active comparators in self-controlled designs. Am J Epidemiol. 2021;190(10):2181–7.
Vouri SM, Jiang X, Morris EJ, Brumback BA, Winterstein AG. Use of negative controls in a prescription sequence symmetry analysis to reduce time-varying bias. Pharmacoepidemiol Drug Saf. 2021;30(9):1192–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
JLJ received the Australian Government Research Training Program (RTP) fee offset scholarship to conduct this study.
Conflict of interest
All authors have no conflicts of interest to declare.
Ethics approval
This study was approved by the External Request Evaluation Committee (EREC) Australian Government Department of Human Services (approval reference number RMS0862).
Consent to participate
Not applicable.
Consent for publication
Consent for publication was obtained from External Request Evaluation Committee (EREC) Australian Government Department of Human Services (approval reference number RMS0862).
Availability of data and materials
Data from Services Australia are available however restrictions apply so are not readily available to the public. A request for data can be submitted to Services Australia.
Code availability
SAS code is available upon request from the corresponding author.
Author contribution
JJ, MW, MS and NP developed and designed the study. NP developed the code and statistical methods to conduct this research. JJ adjusted and utilised the code as necessary to conduct the analysis. JJ wrote the primary manuscript. All authors reviewed and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Janetzki, J.L., Sykes, M.J., Ward, M.B. et al. Chronic Obstructive Pulmonary Disease Adverse Event Signals Associated with Potential Inhibitors of Glutathione Peroxidase 1: A Sequence Symmetry Analysis. Drug Saf 47, 59–70 (2024). https://doi.org/10.1007/s40264-023-01374-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01374-5