Abstract
Introduction
Polypharmacy is common and is associated with higher risk of adverse drug event (ADE) among older adults. Knowledge on the ADE risk level of exposure to different drug combinations is critical for safe polypharmacy practice, while approaches for this type of knowledge discovery are limited. The objective of this study was to apply an innovative data mining approach to discover high-risk and alternative low-risk high-order drug combinations (e.g., three- and four-drug combinations).
Methods
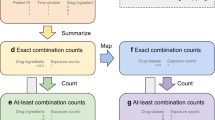
A cohort of older adults (≥ 65 years) who visited an emergency department (ED) were identified from Medicare fee-for-service and MarketScan Medicare supplemental data. We used International Classification of Diseases (ICD) codes to identify ADE cases potentially induced by anticoagulants, antidiabetic drugs, and opioids from ED visit records. We assessed drug exposure data during a 30-day window prior to the ED visit dates. We investigated relationships between exposure of drug combinations and ADEs under the case–control setting. We applied the mixture drug-count response model to identify high-order drug combinations associated with an increased risk of ADE. We conducted therapeutic class-based mining to reveal low-risk alternative drug combinations for high-order drug combinations associated with an increased risk of ADE.
Results
We investigated frequent high-order drug combinations from 8.4 million ED visit records (5.1 million from Medicare data and 3.3 million from MarketScan data). We identified 5213 high-order drug combinations associated with an increased risk of ADE by controlling the false discovery rate at 0.01. We identified 1904 high-order, high-risk drug combinations had potential low-risk alternative drug combinations, where each high-order, high-risk drug combination and its corresponding low-risk alternative drug combination(s) have similar therapeutic classes.
Conclusions
We demonstrated the application of a data mining technique to discover high-order drug combinations associated with an increased risk of ADE. We identified high-risk, high-order drug combinations often have low-risk alternative drug combinations in similar therapeutic classes.

Similar content being viewed by others
References
Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314(17):1818–31. https://doi.org/10.1001/jama.2015.13766.
Qato DM, Wilder J, Schumm P, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473–82. https://doi.org/10.1001/jamainternmed.2015.8581.
Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57–65. https://doi.org/10.1517/14740338.2013.827660.
Becker ML, Kallewaard M, Caspers PWJ, Visser LE, Leufkens HGM, Stricker BH. Hospitalisations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16(6):641–51. https://doi.org/10.1002/pds.1351.
Dechanont S, Maphanta S, Butthum B, Kongkaew C. Hospital admissions/visits associated with drug-drug interactions: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2014;23(5):489–97. https://doi.org/10.1002/pds.3592.
Magro L, Moretti U, Leone R. Epidemiology and characteristics of adverse drug reactions caused by drug-drug interactions. Expert Opin Drug Saf. 2012;11(1):83–94. https://doi.org/10.1517/14740338.2012.631910.
Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA. 2016;316(20):2115–25. https://doi.org/10.1001/jama.2016.16201.
Leonard CE, Brensinger CM, Bilker WB, Kimmel SE, Han X, Nam YH, Gagne JJ, Mangaali MJ, Hennessy S. Gastrointestinal bleeding and intracranial hemorrhage in concomitant users of warfarin and antihyperlipidemics. Int J Cardiol. 2017;228:761–70. https://doi.org/10.1016/j.ijcard.2016.11.245.
Bykov K, Schneeweiss S, Glynn R, Mittleman MA, Gagne JJ. A case-crossover-based screening approach to identifying clinically relevant drug-drug interactions in electronic healthcare data. Clin Pharmacol Ther. 2019;106(1):238–44. https://doi.org/10.1002/cpt.1376.
Han X, Chiang CW, Leonard CE, Bilker WB, Brensinger CM, Li L, Hennessy S. Biomedical Informatics approaches to identifying drug-drug interactions application to insulin secretagogues. Epidemiology. 2017;28(3):459–68. https://doi.org/10.1097/Ede.0000000000000638.
Leonard CE, Bilker WB, Brensinger CM, Han X, Flory JH, Flockhart DA, Gagne JJ, Cardillo S, Hennessy S. Severe hypoglycemia in users of sulfonylurea antidiabetic agents and antihyperlipidemics. Clin Pharmacol Ther. 2016;99(5):538–47. https://doi.org/10.1002/cpt.297.
Nam YH, Brensinger CM, Bilker WB, Leonard CE, Han X, Hennessy S. Serious hypoglycemia and use of warfarin in combination with sulfonylureas or metformin. Clin Pharmacol Ther. 2019;105(1):210–8. https://doi.org/10.1002/cpt.1146.
Abrahamsson T, Berge J, Ojehagen A, Hakansson A. Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment-A nation-wide register-based open cohort study. Drug Alcohol Depend. 2017;174:58–64. https://doi.org/10.1016/j.drugalcdep.2017.01.013.
Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261–72. https://doi.org/10.1111/jgs.13153.
Nobili A, Pasina L, Tettamanti M, Lucca U, Riva E, Marzona I, Monesi L, Cucchiani R, Bortolotti A, Fortino I, Merlino L, Locatelli GW, Giuliani G. Potentially severe drug interactions in elderly outpatients: results of an observational study of an administrative prescription database. J Clin Pharm Ther. 2009;34(4):377–86. https://doi.org/10.1111/j.1365-2710.2009.01021.x.
Qato DM, Ozenberger K, Olfson M. Prevalence of prescription medications with depression as a potential adverse effect among adults in the United States. JAMA. 2018;319(22):2289–98. https://doi.org/10.1001/jama.2018.6741.
Chiang CW, Zhang PY, Wang XY, Wang L, Zhang SJ, Ning X, Shen L, Quinney SK, Li L. Translational high-dimensional drug interaction discovery and validation using health record databases and pharmacokinetics models. Clin Pharmacol Ther. 2018;103(2):287–95. https://doi.org/10.1002/cpt.914.
CDC. Health, United States; 2018 [01/25/2020]. https://www.cdc.gov/nchs/data/hus/2018/038.pdf.
John R, Philip D. Although triple drug interactions are not well studied, available information points to some general principles that may be useful in managing such combination. Pharmacy Times; 2011 [01/25/2020]. https://www.pharmacytimes.com/publications/issue/2011/January2011/Interactions-0111.
Harpaz R, Chase HS, Friedman C. Mining multi-item drug adverse effect associations in spontaneous reporting systems. BMC Bioinform. 2010;11(Suppl 9):S7. https://doi.org/10.1186/1471-2105-11-S9-S7.
Xiang Y, Albin A, Ren K, Zhang P, Etter JP, Lin S, Li L. Efficiently mining Adverse Event Reporting System for multiple drug interactions. AMIA Jt Summits Transl Sci Proc. 2014;2014:120–5.
Chasioti D, Yao X, Zhang P, Lerner S, Quinney SK, Ning X, Li L, Shen L. Mining directional drug interaction effects on myopathy using the FAERS database. IEEE J Biomed Health Inform. 2019;23(5):2156–63. https://doi.org/10.1109/JBHI.2018.2874533.
Wang X, Zhang P, Chiang CW, Wu H, Shen L, Ning X, Zeng D, Wang L, Quinney SK, Feng W, Li L. Mixture drug-count response model for the high-dimensional drug combinatory effect on myopathy. Stat Med. 2018;37(4):673–86. https://doi.org/10.1002/sim.7545.
CMS. Medicare Claims Processing Manual 2019 [01/25/2020]. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R1139CP.pdf.
RxNorm. https://www.nlm.nih.gov/research/umls/rxnorm/index.html.
U.S. Department of Health and Human Services OoDPaHP. National Action Plan for Adverse Drug Event Prevention. Washington, DC; 2014.
Digmann R, Thomas A, Peppercorn S, Ryan A, Zhang L, Irby K, Brock J. Use of medicare administrative claims to identify a population at high risk for adverse drug events and hospital use for quality improvement. J Manag Care Spec Pharm. 2019;25(3):402–10. https://doi.org/10.18553/jmcp.2019.25.3.402.
Wedemeyer RS, Blume H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: an update. Drug Saf. 2014;37(4):201–11. https://doi.org/10.1007/s40264-014-0144-0.
Wintzell V, Svanstrom H, Pasternak B. Selection of comparator group in observational drug safety studies: alternatives to the active comparator new user design. Epidemiology. 2022;33(5):707–14. https://doi.org/10.1097/EDE.0000000000001521.
Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015;11(7):437–41. https://doi.org/10.1038/nrrheum.2015.30.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work is supported by NIH under R01AG071018.
Conflicts of interest
All authors declare no conflict of interest.
Ethics approval
This retrospective observational study was approved by the Institutional Review Board (IRB) at The Ohio State University (ID: 2020H0546).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability
MarketScan data is available from IBM, and Medicare data is available from Center of Medicare and Medicaid Services (CMS).
Code availability
Sample codes can be found in Appendix.
Author contributions
PZ conceived the study; YS and CW conducted data analysis; YS, CW, MD, and PZ drafted the manuscript; KH, KU, JC, AS, YY, LL, MD, and PZ interpreted the results; All authors critically revised and gave final approval of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, Y., Chiang, CW., Unroe, K.T. et al. Application of an Innovative Data Mining Approach Towards Safe Polypharmacy Practice in Older Adults. Drug Saf 47, 93–102 (2024). https://doi.org/10.1007/s40264-023-01370-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01370-9