Abstract
Introduction
In administrative data, accurate timing of exposure relative to gestation is critical for determining the effect of potential teratogen exposure on pregnancy outcomes.
Objective
To develop an algorithm for identifying stillbirth episodes in the ICD-9-CM era using national Medicaid claims data (1999–2014).
Methods
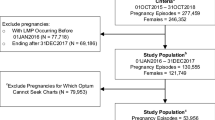
Unique stillbirth episodes were identified from clusters of medical claims using a hierarchy that identified the encounter with the highest potential of including the actual stillbirth delivery and that delineated subsequent pregnancy episodes. Each episode was validated using clinical detail on retrieved medical records as the gold standard.
Results
Among 220 retrieved records, 197 were usable for validation of 1417 stillbirth episodes identified by the algorithm. The positive predictive value (PPV) was 64.0% (57.3–70.7%) overall, 80.4% (73.8–87.1%) for inpatient episodes, 28.2% (14.1–42.3%) for outpatient-only episodes, and 20.0% (2.5–37.5%) for outpatient episodes with overlapping hospitalizations. The absolute difference between the dates of the algorithm-specified stillbirth delivery and the medical record-based event was 4.2 ± 24.3 days overall, 1.7 ± 7.7 days for inpatient episodes, 14.3 ± 51.4 days for outpatient-only episodes, and 1.0 ± 2.0 days for outpatient episodes that overlapped with a hospitalization. Excluding all outpatient episodes, as well as pregnancies involving multiple births, the PPV increased to 82.7% (76.8–89.8%).
Conclusions
Our algorithm to identify stillbirths from administrative claims data had a moderately high PPV. Positive predictive value was substantially increased by restricting the setting to inpatient episodes and using only input diagnostic codes for singleton stillbirths.


Similar content being viewed by others
References
Hornbrook MC, Hurtado AV, Johnson RE. Health care episodes: definition, measurement and use. Med Care Rev. 1985;42(2):163–218.
Zhu Y, Hampp C, Wang X, Albogami Y, Wei YJ, Brumback BA, et al. Validation of algorithms to estimate gestational age at birth in the Medicaid Analytic eXtract-Quantifying the misclassification of maternal drug exposure during pregnancy. Pharmacoepidemiol Drug Saf. 2020;29(11):1414–22.
Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernández-Díaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf. 2013;22(1):16–24.
Li Q, Andrade SE, Cooper WO, Davis RL, Dublin S, Hammad TA, et al. Validation of an algorithm to estimate gestational age in electronic health plan databases. Pharmacoepidemiol Drug Saf. 2013;22(5):524–32.
Eworuke E, Hampp C, Saidi A, Winterstein AG. An algorithm to identify preterm infants in administrative claims data. Pharmacoepidemiol Drug Saf. 2012;21(6):640–50.
Fitzpatrick T, Wilton AS, Guttmann A. Development and validation of a simple algorithm to estimate common gestational age categories using standard administrative birth record data in Ontario, Canada. J Obstet Gynaecol. 2021;41(2):207–11.
Hornbrook MC, Whitlock EP, Berg CJ, Callaghan WM, Bachman DJ, Gold R, et al. Development of an algorithm to identify pregnancy episodes in an integrated health care delivery system. Health Serv Res. 2007;42(2):908–27.
Manson JM, McFarland B, Weiss S. Use of an automated database to evaluate markers for early detection of pregnancy. Am J Epidemiol. 2001;154(2):180–7.
Matcho A, Ryan P, Fife D, Gifkins D, Knoll C, Friedman A. Inferring pregnancy episodes and outcomes within a network of observational databases. PLoS ONE. 2018;13(2): e0192033.
Bertoia ML, Phiri K, Clifford CR, Doherty M, Zhou L, Wang LT, et al. Identification of pregnancies and infants within a US commercial healthcare administrative claims database. Pharmacoepidemiol Drug Saf. 2022;31(8):863–74.
Moll K, Wong HL, Fingar K, Hobbi S, Sheng M, Burrell TA, et al. Validating claims-based algorithms determining pregnancy outcomes and gestational age using a linked claims-electronic medical record database. Drug Saf. 2021;44(11):1151–64.
Minassian C, Williams R, Meeraus WH, Smeeth L, Campbell OMR, Thomas SL. Methods to generate and validate a Pregnancy Register in the UK Clinical Practice Research Datalink primary care database. Pharmacoepidemiol Drug Saf. 2019;28(7):923–33.
Andrade SE, Shinde M, Moore Simas TA, Bird ST, Bohn J, Haynes K, et al. Validation of an ICD-10-based algorithm to identify stillbirth in the Sentinel system. Pharmacoepidemiol Drug Saf. 2021;30(9):1175–83.
Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data for 2019. Natl Vital Stat Rep. 2021;70(2):1–51.
Winterstein AG, Thai TN, Nduaguba S, Smolinski NE, Wang X, Sahin L, et al. Risk of fetal or neonatal death or neonatal intensive care unit admission associated with gadolinium magnetic resonance imaging exposure during pregnancy. Am J Obstet Gynecol. 2022. https://doi.org/10.1016/j.ajog.2022.10.005.
Li Y, Zhu Y, Chen C, Wang X, Choi Y, Henriksen C, et al. Internal validation of Medicaid Analytic eXtract (MAX) data capture for comprehensive managed care plan enrollees from 2007 to 2010. Pharmacoepidemiol Drug Saf. 2018;27(10):1067–76.
Likis FE, Sathe NA, Carnahan R, McPheeters ML. A systematic review of validated methods to capture stillbirth and spontaneous abortion using administrative or claims data. Vaccine. 2013;31(Suppl 10):K74-82.
Jones RK, Jerman J. Abortion incidence and service availability in the United States, 2014. Perspect Sex Reprod Health. 2017;49(1):17–27.
Duke CW, Correa A, Romitti PA, Martin J, Kirby RS. Challenges and priorities for surveillance of stillbirths: a report on two workshops. Public Health Rep. 2009;124(5):652–9.
Hoyert DL, Gregory ECW. Cause-of-death data from the fetal death file, 2015–2017. Natl Vital Stat Rep. 2020;69(4):1–20.
Thai TN, Rasmussen SA, Smolinski NE, Nduaguba S, Zhu Y, Bateman BT, et al. Implication of maternal continuous enrollment on stillbirth gestational age distributions and maternal characteristics in Medicaid enrollees. Am J Epidemiol. 2022. https://doi.org/10.1093/aje/kwac206.
Palmsten K, Huybrechts KF, Mogun H, Kowal MK, Williams PL, Michels KB, et al. Harnessing the Medicaid Analytic eXtract (MAX) to evaluate medications in pregnancy: design considerations. PLoS ONE. 2013;8(6): e67405.
Adam MP, Polifka JE, Friedman JM. Evolving knowledge of the teratogenicity of medications in human pregnancy. Am J Med Genet C Semin Med Genet. 2011;157C(3):175–82.
Suarez EA, Bateman BT, Hernández-Díaz S, Straub L, Wisner KL, Gray KJ, et al. Association of antidepressant use during pregnancy with risk of neurodevelopmental disorders in children. JAMA Intern Med. 2022;182(11):1149–60.
Bateman BT, Hernandez-Diaz S, Straub L, Zhu Y, Gray KJ, Desai RJ, et al. Association of first trimester prescription opioid use with congenital malformations in the offspring: population based cohort study. BMJ. 2021;372: n102.
Thai TN, Sarayani A, Wang X, Albogami Y, Rasmussen SA, Winterstein AG. Risk of pregnancy loss in patients exposed to mycophenolate compared to azathioprine: a retrospective cohort study. Pharmacoepidemiol Drug Saf. 2020;29(6):716–24.
Huybrechts KF, Bateman BT, Zhu Y, Straub L, Mogun H, Kim SC, et al. Hydroxychloroquine early in pregnancy and risk of birth defects. Am J Obstet Gynecol. 2021;224(3):290.e1-290.e22.
Acknowledgements
The authors thank the Florida Department of Health for provision of birth and fetal death certificates. This study represents the opinions of the authors and not necessarily those of the Food and Drug Administration or the Florida Department of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Food and Drug Administration (contract HHSF223201810083C).
Conflict of interest
SON, NES, and TNT have no conflict of interest to declare. STB works for the US Food and Drug Administration and have no conflicts of interest to disclose. SAR has served on an advisory committee for the Teva Pregnancy Registry; has consulted for F. Hoffmann-La Roche AG as a litigation consultant; and receives grant support from the National Institutes of Health, and the Centers for Disease Control and Prevention, and Health Services Research Administration. AGW has received funding for research studies unrelated to this work from NIH, AHRQ, PCORI, FDA, the Bill and Melinda Gates Foundation, CDC, Merck Sharpe and Dohme, and the state of Florida. She has received consulting fees from Arbor Pharmaceuticals, Bayer, Ipsen, and Genentech Inc., likewise unrelated to this work.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
The data that support the findings of this study are available from the Centers for Medicaid and Medicare Services but restrictions apply to the availability of these data, which were used under a data user agreement for the current study and are not publicly available.
Code availability
Codes will be made available upon request.
Author contributions
SON made substantial contribution to the design, analysis, and interpretation of data; as well as drafting the manuscript. NES made substantial contribution to the design, analysis, and interpretation of data and also contributed to the critical revision of the manuscript. TNT made substantial contribution to the design and interpretation of data and also contributed to the critical revision of the manuscript. STB made substantial contribution to the interpretation of data and also contributed to the critical revision of the manuscript. SAR made substantial contribution to the design and interpretation of data and also contributed to the critical revision of the manuscript. AGW made substantial contribution to the conception and design, data acquisition, analysis, and interpretation of data; as well as the critical revision of the manuscript. All authors read and approved the final version.
FDA disclaimer
This work represents the opinions of the authors and not necessarily those of the Food and Drug Administration.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nduaguba, S.O., Smolinski, N.E., Thai, T.N. et al. Validation of an ICD-9-Based Algorithm to Identify Stillbirth Episodes from Medicaid Claims Data. Drug Saf 46, 457–465 (2023). https://doi.org/10.1007/s40264-023-01287-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01287-3