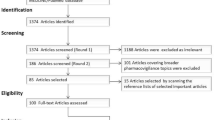
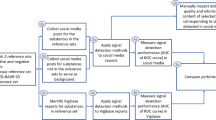
Abstract
The large-scale use of social media by the population has gained the attention of stakeholders and researchers in various fields. In the domain of pharmacovigilance, this new resource was initially considered as an opportunity to overcome underreporting and monitor the safety of drugs in real time in close connection with patients. Research is still required to overcome technical challenges related to data extraction, annotation, and filtering, and there is not yet a clear consensus concerning the systematic exploration and use of social media in pharmacovigilance. Although the literature has mainly considered signal detection, the potential value of social media to support other pharmacovigilance activities should also be explored. The objective of this paper is to present the main findings and subsequent recommendations from the French research project Vigi4Med, which evaluated the use of social media, mainly web forums, for pharmacovigilance activities. This project included an analysis of the existing literature, which contributed to the recommendations presented herein. The recommendations are categorized into three categories: ethical (related to privacy, confidentiality, and follow-up), qualitative (related to the quality of the information), and quantitative (related to statistical analysis). We argue that the progress in information technology and the societal need to consider patients’ experiences should motivate future research on social media surveillance for the reinforcement of classical pharmacovigilance.
Similar content being viewed by others
References
Medawar C, Herxheimer A, Bell A, Jofre S. Paroxetine, Panorama and user reporting of ADRs: consumer intelligence matters in clinical practice and post-marketing drug surveillance. Int J Risk Saf Med. 2002;15(4/3):161–9.
Curino CA, Jia Y, Lambert B, West PM, Yu C. Mining officially unrecognized side effects of drugs by combining web search and machine learning. In: Proceedings of the 14th ACM international conference on Information and knowledge management. 2005. p. 365–72.
Schröder S, Zöllner YF, Schaefer M. Drug related problems with Antiparkinsonian agents: consumer internet reports versus published data. Pharmacoepidemiol Drug Saf. 2007;16(10):1161–6.
Scanfeld D, Scanfeld V, Larson EL. Dissemination of health information through social networks: Twitter and antibiotics. Am J Infect Control. 2010;38(3):182–8.
Leaman R, Wojtulewicz L, Sullivan R, Skariah A, Yang J, Gonzalez G. Towards internet-age pharmacovigilance: extracting adverse drug reactions from user posts to health-related social networks. In: Proceedings of the 2010 workshop on biomedical natural language processing. Association for Computational Linguistics, 2010. p. 117–25.
Golder S, Norman G, Loke YK. Systematic review on the prevalence, frequency and comparative value of adverse events data in social media. Br J Clin Pharmacol. 2015;80(4):878–88.
Sloane R, Osanlou O, Lewis D, Bollegala D, Maskell S, Pirmohamed M. Social media and pharmacovigilance: a review of the opportunities and challenges. Br J Clin Pharmacol. 2015;80(4):910–20.
Lardon J, et al. Adverse drug reaction identification and extraction in social media: a scoping review. J Med Internet Res. 2015;17(7):1–16.
Convertino I, Ferraro S, Blandizzi C, Tuccori M. The usefulness of listening social media for pharmacovigilance purposes: a systematic review. Expert Opin Drug Saf. 2018;17(11):1081–93.
Tricco AC, et al. Utility of social media and crowd-intelligence data for pharmacovigilance: a scoping review. BMC Med Inform Decis Mak. 2018;18(1):1–14.
Pappa D, Stergioulas LK. Harnessing social media data for pharmacovigilance: a review of current state of the art, challenges and future directions. Int J Data Sci Anal. 2019;8:113–35.
Micoulaud-Franchi JA. Un pas de plus vers une pharmacovigilance 2.0. Intégration des données du web communautaire à une pharmacovigilance plus alerte. Press Medicale. 2011;40(9):790–2.
Seifert HA, et al. Enabling social listening for cardiac safety monitoring: Proceedings from a drug information association-cardiac safety research consortium cosponsored think tank. Am Heart J. 2017;194:107–15.
Bousquet C, et al. The adverse drug reactions from patient reports in social media project: five major challenges to overcome to operationalize analysis and efficiently support pharmacovigilance process. JMIR Res Protoc. 2017;6(9):e179.
Kierzek G, Leo M. Rapport sur l’amélioration de l’information des usagers et des professionnels de santé sur le médicament. Mission report 2018 [Online]. https://solidarites-sante.gouv.fr/IMG/pdf/180903_-_mim_rapport.pdf. Accessed May 2020.
European Medicines Agency. Guideline on good pharmacovigilance practices (GVP)—Module VI—collection, management and submission of reports of suspected adverse reactions to medicinal products (Rev 2). London: EMA; 2017.
European Medicines Agency. HMA-EMA Joint Big Data Taskforce-summary report Summary report. London: EMA; 2019.
Ghosh R, Lewis D. Aims and approaches of Web-RADR: a consortium ensuring reliable ADR reporting via mobile devices and new insights from social media. Expert Opin Drug Saf. 2015;14(12):1845–53.
Caster O, et al. Assessment of the utility of social media for broad-ranging statistical signal detection in pharmacovigilance: results from the WEB-RADR Project. Drug Saf. 2018;41(12):1355–69.
van Stekelenborg J, et al. Recommendations for the use of social media in pharmacovigilance: lessons from IMI WEB-RADR. Drug Saf. 2019;42(12):1393–407.
Karapetiantz P, et al. Descriptions of adverse drug reactions are less informative in forums than in the French Pharmacovigilance database but provide more unexpected reactions. Front Pharmacol. 2018;9:1–11.
Audeh B, Beigbeder M, Zimmermann A, Jaillon P, Bousquet CD. “Vigi4Med Scraper: a framework for web forum structured data extraction and semantic representation. PLoS One. 2017;12(1):e0169658.
Morlane-Hondère F, Grouin C, Zweigenbaum P. Identification of drug-related medical conditions in social media. In: Proceedings of the Tenth International Conference on Language Resources and Evaluation (LREC'16). 2016. p. 2022–8.
Karapetiantz P, Audeh B, Louët AL-L, Bousquet C. Signal detection for baclofen in web forums: a preliminary study. Stud Health Technol Inform. 2018;247:421–5.
Audeh B, et al. Pharmacology and social media: Potentials and biases of web forums for drug mention analysis—case study of France. Health Inform J. 2019. https://doi.org/10.1177/1460458219865128.
Bate A, Reynolds RF, Caubel P. The hope, hype and reality of Big Data for pharmacovigilance. Ther Adv Drug Saf. 2018;9(1):5–11.
Audeh B et al. French Levothyrox® crisis: retrospective analysis of social media. In: International Society of Pharmacovigilance. 2019.
Lardon J, et al. Evaluating Twitter as a complementary data source for pharmacovigilance. Expert Opin Drug Saf. 2018;17(8):763–74.
Edwards IR, Lindquist M, Wiholm BE, Napke E. Quality criteria for early signals of possible adverse drug reactions. Lancet. 1990;336(8708):156–8.
The european parliament and the council of the european union. Regulation (eu) 2016/679 of the european parliament and of the council of 27 april 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing directive 95/46/ec (general da, Official Journal of the European Union, 2016. [Online]. https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX:32016R0679. Accessed May 2020.
Golder S, Scantlebury A, Christmas H. Understanding public attitudes toward researchers using social media for detecting and monitoring adverse events data: multi methods study. Journal of medical Internet research. 2019;21(8):e7081.
Lengsavath M, et al. Social media monitoring and adverse drug reaction reporting in pharmacovigilance: an overview of the regulatory landscape. Ther Innov Regul Sci. 2017;51(1):125–31.
Naik P, et al. Regulatory definitions and good pharmacovigilance practices in social media: challenges and recommendations. Ther Innov Regul Sci. 2015;49(6):840–51.
Brosch S, de Ferran AM, Newbould V, Farkas D, Lengsavath M, Tregunno P. Establishing a framework for the use of social media in pharmacovigilance in Europe. Drug Saf. 2019;42:921–30.
Azam R. Accessing social media information for pharmacovigilance: what are the ethical implications? Ther Adv Drug Saf. 2018;9(6):259–61.
Kheloufi F, Default A, Blin O, Micallef J. Investigating patient narratives posted on Internet and their informativeness level for pharmacovigilance purpose: the example of comments about statins. Therapie. 2017;72(4):483–90.
Sadah SA, Shahbazi M, Wiley MT, Hristidis V. Demographic-based content analysis of web-based health-related social media. J Med Internet Res. 2016;18(6):1–13.
Sinclair M, Lagan BM, Dolk H, McCullough JEM. An assessment of pregnant women’s knowledge and use of the Internet for medication safety information and purchase. J Adv Nurs. 2018;74(1):137–47.
Keller MS, Mosadeghi S, Cohen ER, Kwan J, Spiegel BMR. Reproductive health and medication concerns for patients with inflammatory bowel disease: thematic and quantitative analysis using social listening. J Med Internet Res. 2018;20(6):e206.
Rezaallah B, Lewis DJ, Pierce C, Zeilhofer HF, Berg BI. Social media surveillance of multiple sclerosis medications used during pregnancy and breastfeeding: thematic qualitative analysis. J Med Internet Res. 2019;21(8):e13003.
Bigeard E, Grabar N, Thiessard F. Detection and analysis of drug misuses. A study based on social media messages. Front Pharmacol. 2018;9:1–16.
Zhao M, Yang CC. Automated off-label drug use detection from user generated content. In: Proceedings of the 8th ACM International Conference on Bioinformatics, Computational Biology, and Health Informatics. 2017. p. 449–54.
Campillos-llanos L, Grouin C, Louët AL, Zweigenbaum P. Initial experiments for pharmacovigilance analysis in social media using summaries of product characteristics. Stud Health Technol Inform. 2019;264:60–64.
Cameron D, et al. PREDOSE: a semantic web platform for drug abuse epidemiology using social media. J Biomed Inform. 2013;46(6):985–97.
Zhao M, Yang CC. Exploiting OHC data with tensor decomposition for off-label drug use detection. In: 2018 IEEE International Conference on Healthcare Informatics (ICHI). IEEE, 2018. p. 22–8.
Bigeard É, Thiessard F, Grabar N. Detecting drug non-compliance in internet fora using information retrieval and machine learning approaches. Stud Health Technol Inform. 2019;264:30–4.
Sarker A, et al. Social media mining for toxicovigilance: automatic monitoring of prescription medication abuse from twitter. Drug Saf. 2016;39(3):231–40.
Anderson L, et al. Using social listening data to monitor misuse and nonmedical use of bupropion: a content analysis. JMIR Public Heal Surveill. 2017;3(1):e6.
Sarker A, DeRoos A, Perrone J. Mining social media for prescription medication abuse monitoring: a review and proposal for a data-centric framework. J Am Med Inform Assoc. 2020;27(2):315–29.
Abdellaoui R, Foulquie P, Texier N, Faviez C, Burgun A, Schück S. Detection of cases of noncompliance to drug treatment in patient forum posts: topic model approach. J Med Internet Res. 2018;20(3):1–12.
Rees S, Mian S, Grabowski N. Using social media in safety signal management: is it reliable? Ther Adv Drug Saf. 2018;9(10):591–9.
Patel R, et al. Frequent discussion of insomnia and weight gain with glucocorticoid therapy: an analysis of Twitter posts. NPJ Digit Med. 2018;1(1):20177.
Park SH, Hong SH. Identification of primary medication concerns regarding thyroid hormone replacement therapy from online patient medication reviews: text mining of social network data. J Med Internet Res. 2018;20(10):1–10.
Isah H, Trundle P, Neagu D. Social media analysis for product safety using text mining and sentiment analysis. In: Proceedings 2014 14th UK Work Comput Intell (UKCI). IEEE, 2014. p. 1–7.
Antipov EA, Pokryshevskaya EB. The effects of adverse drug reactions on patients’ satisfaction: evidence from publicly available data on Tamiflu (oseltamivir). Int J Med Inform. 2019;125:30–6.
Bousquet C, Audeh B, Bellet F, Louët AL-L. Comment on: ‘Assessment of the Utility of Social Media for Broad-Ranging Statistical Signal Detection in Pharmacovigilance: results from the WEB-RADR Project. Drug Saf. 2018;41(12):1355–69.
Moncrieff J, Cohen D, Mason JP. The subjective experience of taking antipsychotic medication: a content analysis of Internet data. Acta Psychiatr Scand. 2009;120(2):102–11.
Du J, Xu J, Song HY, Tao C. Leveraging machine learning-based approaches to assess human papillomavirus vaccination sentiment trends with Twitter data. BMC Med Inform Decis Mak. 2017;17(Suppl 2):69.
Booth A, et al. Using social media to uncover treatment experiences and decisions in patients with acute myeloid leukemia or myelodysplastic syndrome who are ineligible for intensive chemotherapy: patient-centric qualitative data analysis. J Med Internet Res. 2019;21(11):1–12.
Golomb BA, Mcgraw JJ, Evans MA, Dimsdale JE. Physician response to patient reports. Drug Saf. 2007;30(8):669–75.
Vaughan Sarrazin MS, Cram P, Mazur A, Ward M, Reisinger HS. Patient perspectives of dabigatran: analysis of online discussion forums. Patient. 2014;7(1):47–544.
Abou Taam M, et al. Analysis of patients’ narratives posted on social media websites on benfluorex’s (Mediator®) withdrawal in France. J Clin Pharm Ther. 2014;39(1):53–5.
Bhattacharya M, et al. Using social media data in routine pharmacovigilance: a pilot study to identify safety signals and patient perspectives. Pharmaceut Med. 2017;31(3):167–74.
Topaz M, et al. Clinicians’ reports in electronic health records versus patients’ concerns in social media: a pilot study of adverse drug reactions of aspirin and atorvastatin. Drug Saf. 2015;39(3):243–50.
Tafti AP, et al. Adverse drug event discovery using biomedical literature: a big data neural network adventure. JMIR Med Inform. 2017;5(4):e51.
Smith K, Golder S, Sarker A, Loke Y, O’Connor K, Gonzalez-Hernandez G. Methods to compare adverse events in Twitter to FAERS, drug information databases, and systematic reviews: proof of concept with adalimumab. Drug Saf. 2018;41(12):1397–410.
Nikfarjam A, et al. Early detection of adverse drug reactions in social health networks: a natural language processing pipeline for signal detection. JMIR Public Heal Surveill. 2019;5(2):e11264.
Duh MS, et al. Can social media data lead to earlier detection of drug-related adverse events? Pharmacoepidemiol Drug Saf. 2016;25(12):1425–33.
Gavrielov-Yusim N, et al. Comparison of text processing methods in social media–based signal detection. Pharmacoepidemiol. Drug Saf. 2019;28(10):1309–17.
Wu H, Fang H, Stanhope SJ. Exploiting online discussions to discover unrecognized drug side effects. Methods Inf Med. 2013;52(2):152–9.
Yang H, Yang CC. Using health-consumer-contributed data to detect adverse drug reactions by association mining with temporal analysis. ACM Trans Intell Syst Technol. 2015;6(4):55.
Feldman R, Netzer O, Peretz A, Rosenfeld B. Utilizing text mining on online medical forums to predict label change due to adverse drug reactions. In: Proceedings of the 21th ACM SIGKDD international conference on knowledge discovery and data mining (KDD), 2015. p. 1779–88.
Ransohoff JD, et al. Detecting chemotherapeutic skin adverse reactions in social health networks using deep learning. JAMA Oncol. 2018;4(4):581–3.
Golder S, et al. Pharmacoepidemiologic evaluation of birth defects from health-related postings in social media during pregnancy. Drug Saf. 2019;42(3):389–400.
Pinheiro LC, Candore G, Zaccaria C, Slattery J, Arlett P. An algorithm to detect unexpected increases in frequency of reports of adverse events in EudraVigilance. Pharmacoepidemiol Drug Saf. 2018;27(1):38–45.
Trinh NTH, Solé E, Benkebil M. Benefits of combining change-point analysis with disproportionality analysis in pharmacovigilance signal detection. Pharmacoepidemiol Drug Saf. 2019;28(3):370–6.
Barry D, Hartigan JA. A Bayesian analysis for change point problems. J Am Stat Assoc. 1993;88(421):309–19.
Butt TF, Cox AR, Oyebode JR, Ferner RE. Internet accounts of serious adverse drug reactions. Drug Saf. 2012;35(12):1159–70.
Comfort S, et al. Sorting through the safety data haystack: using machine learning to identify individual case safety reports in social-digital media. Drug Saf. 2018;41(6):579–90.
Cocos A, Fiks AG, Masino AJ. Deep learning for pharmacovigilance: recurrent neural network architectures for labeling adverse drug reactions in Twitter posts. J Am Med Inform Assoc. 2017;24(4):813–21.
Sarker A, et al. Data and systems for medication-related text classification and concept normalization from Twitter: insights from the Social Media Mining for Health (SMM4H)-2017 shared task. J Am Med Inform Assoc. 2018;25(10):1274–83.
Chee BW, Berlin R, Schatz B. Predicting adverse drug events from personal health messages. In: AMIA Annual Symposium Proceedings. American Medical Informatics Association, 2011. p. 217.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by the AAP-2013-052 grant from the French agency for drug safety, Agence nationale de sécurité du médicament et des produits de santé (ANSM), through the Vigi4Med research project, and Convention no 2016S076 through the PHARES project. The views expressed in this article are those of the authors and do not necessarily represent the views of the ANSM.
Conflict of interest
Bissan Audeh, Florelle Bellet, Marie-Noëlle Beyens, Agnès Lillo-Le Louët, and Cédric Bousquet have no conflicts of interest that are directly relevant to the content of this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Audeh, B., Bellet, F., Beyens, MN. et al. Use of Social Media for Pharmacovigilance Activities: Key Findings and Recommendations from the Vigi4Med Project. Drug Saf 43, 835–851 (2020). https://doi.org/10.1007/s40264-020-00951-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-020-00951-2