Abstract
Introduction
Medicine safety signal detection methods employed by the medicine regulator in Australia (Therapeutic Goods Administration [TGA], Department of Health) rely predominantly on analysis of spontaneous adverse event (AE) reports, sponsor notifications or information shared by international agencies. The limitations of these methods and the availability of large administrative health data sets has given rise to greater interest in the use of administrative health data to support pharmacovigilance (PV).
Objective
We explored whether prescription sequence symmetry analysis (PSSA) of Pharmaceutical Benefits Scheme (PBS) data can enhance signal detection by the TGA, using the AE, heart failure (HF) as a case study.
Methods
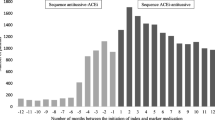
We applied the PSSA method to all single-ingredient medicines dispensed under the PBS between 2012 and 2016, using furosemide initiation as a proxy for new-onset HF. A signal was considered present if the lower limit of the 95% confidence interval for the adjusted sequence ratio was > 1. We excluded medicines known to cause HF, indicated for HF treatment or indicated for diseases that may contribute to HF.
Results
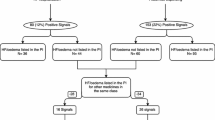
Of the 654 tested medicines, 26 potential new HF signals were detected by PSSA. Five signals had additional support for the possible association provided by biological plausibility, consistency and disproportionate reporting of cases of HF to the TGA and the World Health Organization; and clinical impact.
Conclusion
PSSA was able to identify potential signals for further evaluation. With the increasing availability of different administrative health data sources, the strengths and weaknesses of methods used to analyse these data for the purpose of regulatory PV should be evaluated.


Similar content being viewed by others
References
World Health Organization. Quality Assurance and Safety of Medicines Team. Safety of medicines: a guide to detecting and reporting adverse drug reactions : why health professionals need to take action. Geneva: World Health Organization; 2002. https://apps.who.int/iris/handle/10665/67378. Accessed 20 June 2018.
Arnaud M, Begaud B, Thurin N, et al. Methods for safety signal detection in healthcare databases: a literature review. Expert Opin Drug Saf. 2017;16(6):721–32.
Mellish L, Karanges EA, Litchfield MJ, Schaffer AL, Blanch B, Daniels BJ, et al. The Australian Pharmaceutical Benefits Scheme data collection: a practical guide for researchers. BMC Res Notes. 2015;8:634.
New Health Policy Brief: The FDA’s Sentinel Initiative. Health Affairs Blog. 2015.
Coloma PM, Trifiro G, Patadia V, Sturkenboom M. Postmarketing safety surveillance. Where does signal detection using electronic health records fit into the big picture? Drug Saf. 2013;36:183–97.
Patadia VK, Coloma P, Schuemie MJ, Herings R, Gini R, Mazzaglia G, et al. Using real-world healthcare data for pharmacovigilance signal detection—the experience of the EU-ADR project. Expert Rev Pharmacol. 2015;8(1):95–102.
Pratt N, Roughead E. Assessment of medication safety using only dispensing data. Curr Epidemiol Rep. 2018;5(4):357–69.
Roughhead EE, Chan EW, Choi NK, et al. Proton pump inhibitors and risk of clostridium difficile infection: a multi-country study using sequence symmetry analysis. Expert Opin Drug Saf. 2016;15(12):1589–95.
Roughead EE, Chan EW, Choi NK, et al. Variation in association between thiazolidinediones and heart failure across ethnic groups: retrospective analysis of large healthcare claims databases in six countries. Drug Saf. 2015;38(9):823–31.
Wahab IA, Pratt NL, Ellet LK, et al. Sequence symmetry analysis as a signal detection tool for potential heart failure adverse events in an administrative claims database. Drug Saf. 2016;39(4):347–54.
Nishtala PS, Chyou T. Exploring New Zealand prescription data using sequence symmetry analyses for predicting adverse drug reactions. J Clin Pharm Ther. 2017;42:189–94.
World Health Organization collaborating Centre for Drug Statistics Methodology. Anatomical Therapeutic Chemical Code Classification index with Defined Daily Doses 2017. 2017. https://www.whocc.no/atc_ddd_index_and_guidelines/atc_ddd_index/. Accessed 5 Dec 2017.
Australian Government. Department of Health. Schedule of Pharmaceutical Benefits 2017. 2017. https://www.pbs.gov.au/publication/schedule/2017/12/2017-12-01-general-schedule.pdf. Accessed 5 Dec 2017.
Wahab IA, Pratt NL, Wiese MD, et al. The validity of sequence symmetry analysis for adverse drug reaction signal detection. Pharmacoepidemiol Drug Saf. 2013;22:496–502.
Tsiropoulos I, Anndersen M, Hallas J. Adverse events with use of antiepileptic drugs: a prescription and event symmetry analysis. Pharmacoepid and Drug Saf. 2009;18:483–91.
Page RL, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure. A scientific statement from the American Health Association. Circulation. 2016;134:e32–e69.
Han X, Zhou Y, Lu W. Precision cardio-oncology: understanding the cardiotoxicity of cancer therapy. NPJ Precis Oncol. 2017;1(1):31.
Depetris I, et al. Fluoropyrimidine-induced cardiotoxicity. Crit Rev Oncol Hematol. 2018;124:1–10.
Wahab IA, Pratt NL, Kalisch LM, et al. Sequence symmetry analysis and disproportionality analyses: what percentage of adverse drug reactions do they signal? Adv Pharmacoepidemiol Drug Saf. 2013;2(4):1000140.
Hallas J, Wang SV, Gagne JJ, Schneeweiss S, Pratt N, Pottegard A. Hypothesis-free screening of large administrative databases for unsuspected drug-outcome associations. Eur J Epidemiolo. 2018;33:545–55.
Hoang T, Liu J, Roughead E, et al. Supervised signal detection for adverse drug reactions in medication dispensing data. Comput Methods Programs Biomed. 2018;161:25–38.
Pottegård A, Hallas J, Wang SV, Gagne JJ. Identifying signals of interest when screening for drug-outcome associations in health care data. Br J Clin Pharmacol. 2018;84:1865–7.
Page AT, Falster MO, Litchfield M, Pearson SA, Etherton-Beer C. Polypharmacy among older Australians, 2006–2017: a population-based study. Med J Aust. 2019;211:71–5.
Ingrasciotta Y, Cutroneo PM, Marciano I, Giezen T, Atzeni F, Trifiro G. Safety of biologics, including biosimilars: perspectives on current status and future direction. Drug Saf. 2018;41:1013–102.
Lai EC, Yan YK, Lin SJ, Hsieh CY. Use of antiepileptic drugs and risk of hypothyroidism. Pharmacoepid Drug Saf. 2013;22:1071–9.
Himmel W, Kochen MM, Sorns U, et al. Drug changes at the interface between primary and secondary care. Int J Clin Pharmacol Ther. 2004;42(2):103–9.
Pratt N, Andersen M, Bergman U, et al. Multi-country rapid adverse drug event assessment: the Asian Pharmacoepidemiology Network (AsPen) antipsychotic and acute hyperglycaemia study. Pharmacoepidemiol Drug Saf. 2013;22(9):915–24.
Trifiro G, Sultana J, Bate A. From big data to smart data for pharmacovigilance: the role of healthcare databases and other emerging sources. Drug Saf. 2018;41:143–9.
Wahab IA, Pratt NL, Kalisch LM, Roughead EE. Comparing time to adverse drug reaction signals in a spontaneous reporting database and a claims database: a case study of rofecoxib-induced myocardial infarction and rosiglitazone-induced heart failure signals in Australia. Drug Saf. 2014;37:53–64.
Pacurariu AC, Straus SM, Trifiro G, Schuemie MJ, Gini R, et al. Useful interplay between spontaneous ADR reports and electronic healthcare records in signal detection. Drug Saf. 2015;38:1201–10.
Acknowledgements
The authors thank Brigitta Osterberger at the TGA for her help in editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author Contributions
All authors contributed to study conception and design. Data collection and analysis were performed by LT and NC, with the statistical code provided by NLP. Interpretation of the results was performed by CEK, MW, NN, ECB, NLP and LKE. The first draft of the manuscript was written by CEK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability Statement
The list of PSSA signals for furosemide initiation is provided in Appendix A in the Electronic Supplementary Materials. No additional data available. The data that support the findings of this study are available from the Australian Government Department of Health but restrictions apply to the availability of these data, and so are not publicly available.
Funding
NP was funded by a National Health and Medical Research Centre (NHMRC) Grant, Centre of Research Excellence in post-marketing surveillance of medicines and medical devices GNT 1040938 and NHMRC Project Grant GNT 1157506. LKE was supported by an NHMRC-ARC Dementia Research Development Fellowship (Grant identification number APP1101788).
Conflict of interest
All authors have no conflicts of interest that are directly relevant to the content of the manuscript.
Ethics approval
The PBS data fields used in the study were medicine item code, supply date of medicine and patient identification (ID) key. As the patient ID key was a unique data key rather than a number that can be used to re-identify individuals, the research was considered negligible risk under the National Statement on Ethical Conduct in Human Research. The project was discussed with the Department of Health Human Research Ethics Committee, but formal ethics approval was not required.
Statement about prior postings and presentations
The results of the study have not been published previously.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
King, C.E., Pratt, N.L., Craig, N. et al. Detecting Medicine Safety Signals Using Prescription Sequence Symmetry Analysis of a National Prescribing Data Set. Drug Saf 43, 787–795 (2020). https://doi.org/10.1007/s40264-020-00940-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-020-00940-5