Abstract
Introduction
The consequences of the withdrawal of marketing authorisation of drugs have mostly been studied considering drug prescription patterns for the therapeutic alternatives of the withdrawn drugs. The potential concomitant changes in the reporting of adverse reactions concerning these alternatives have been studied less often.
Objective
The objective of this study was to analyse the changes in the reporting of adverse events (AEs) for therapeutic alternatives after the withdrawal of three medicines (dextropropoxyphene, pioglitazone and tetrazepam) from the market for safety reasons.
Methods
This study was performed using both the French pharmacovigilance database and the Echantillon Généraliste des Bénéficiaires (a random sample of French health insurance affiliates). For dextropropoxyphene, pioglitazone and tetrazepam alternatives, the number and types of case reports were studied for both the year preceding the first official safety warning and the year following the withdrawal. Reporting rates expressed per 10,000 reimbursements (RRReimb) and per 10,000 treated patients (RRPat) were also compared for the two periods.
Results
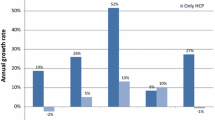
After dextropropoxyphene withdrawal, case reports and reimbursements increased for tramadol (case reports: +23%, reimbursements: +13%) and codeine (case reports: +74%, reimbursements: +47%), RRPat being significantly increased for tramadol (0.92 vs. 1.06, p = 0.02). After pioglitazone withdrawal, case reports increased for dipeptidyl peptidase-4 (DPP-4) inhibitors, glinides, and glucagon-like peptide 1 (GLP-1) analogues (+84%, +22% and +5%, respectively) and reimbursements (+55, +11 and +50%, respectively); both decreased for sulfonylureas (case reports: −6%, reimbursements: −2%). RRPat increased for DPP-4 inhibitors (1.63 vs. 2.26, p = 0.008). After tetrazepam withdrawal, case reports increased for diazepam, methocarbamol and thiocolchicoside (+110, +86 and +157%, respectively), as lesser did reimbursements. RRPat increased for diazepam (1.78 vs. 2.41, p = 0.054) and thiocolchicoside (0.14 vs. 0.24, p = 0.013).
Conclusion
For the three drug withdrawals investigated, the number of case reports involving alternatives increased to a larger extent than the numbers of prescriptions. This could relate to a higher occurrence of AEs in new users of alternatives who switched from the withdrawn medicines or to an increased awareness of possible AEs.
Similar content being viewed by others
Notes
MedDRA® terminology is the international medical terminology developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). The MedDRA® trademark is owned by the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) on behalf of the ICH.
References
Paludetto MN, Olivier-Abbal P, Montastruc JL. Is spontaneous reporting always the most important information supporting drug withdrawals for pharmacovigilance reasons in France? Pharmacoepidemiol Drug Saf. 2012;21(12):1289–94.
ANSM. Retrait de lots et de produits. http://ansm.sante.fr/. Accessed 22 Jan 2016.
Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. BMC Med. 2016;14(1):10.
Bismuth S, Leng EL, Oustric S, Montastruc JL, Lapeyre-Mestre M. Which analgesic after dextropropoxyphene withdrawal? A survey in a sample of general practitioners in southwest of France [in French]. Therapie. 2011;66(1):25–8.
Becquemont L, Delespierre T, Bauduceau B, Benattar-Zibi L, Berrut G, Corruble E, et al. Consequences of dextropropoxyphene market withdrawal in elderly patients with chronic pain. Eur J Clin Pharmacol. 2014;70(10):1237–42.
Hsiao FY, Tsai YW, Huang WF. Changes in physicians’ practice of prescribing cyclooxygenase-2 inhibitor after market withdrawal of rofecoxib: a retrospective study of physician–patient pairs in Taiwan. Clin Ther. 2009;31(11):2618–27.
Aronson JK, Ferner RE. Joining the DoTS: new approach to classifying adverse drug reactions. BMJ. 2003;327(7425):1222–5.
Davidson MH, Clark JA, Glass LM, Kanumalla A. Statin safety: an appraisal from the adverse event reporting system. Am J Cardiol. 2006;97(8A):32C–43C.
Schneeweiss S, Glynn RJ, Avorn J, Mamdani M, Mogun H, Solomon DH. NSAID switching and short-term gastrointestinal outcome rates after the withdrawal of rofecoxib. Pharmacoepidemiol Drug Saf. 2009;18(12):1134–42.
Moore N, Biour M, Paux G, Loupi E, Begaud B, Boismare F, et al. Adverse drug reaction monitoring: doing it the French way. Lancet. 1985;2(8463):1056–8.
Moore N, Noblet C, Kreft-Jais C, Lagier G, Ollagnier M, Imbs JL. French pharmacovigilance database system: examples of utilisation [in French]. Therapie. 1995;50(6):557–62.
Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20(2):109–17.
De Roquefeuil LSA, Neumann A, Merlière Y. L’échantillon généraliste de bénéficiaires : représentativité, portée et limites. Prat Organ Soins. 2009;40(3):213–23.
Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merliere Y. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58(4):286–90.
ANSM. Médicaments contenant du dextropropoxyphène : Retrait progressif de l’AMM – Communiqué du 20/07/2010. http://www.ansm.sante.fr/S-informer/Presse-Communiques-Points-presse/Medicaments-contenant-du-dextropropoxyphene-Retrait-progressif-de-l-AMM-Communique. Accessed 17 Dec 2015.
ANSM. Spécialités contenant du dextropropoxyphène : retrait du marché le 1er mars 2011 - Lettre aux professionnels de santé du 16/02/2011. http://www.ansm.sante.fr/S-informer/Informations-de-securite-Lettres-aux-professionnels-de-sante/Specialites-contenant-du-dextropropoxyphene-retrait-du-marche-le-1er-mars-2011-Lettre-aux-professionnels-de-sante. Accessed 17 Dec 2015.
ANSM. Mise en garde sur l’utilisation de la pioglitazone en traitement chronique chez les patients diabétiques (Actos®, Competact®) – Communiqué du 19/04/2011. http://ansm.sante.fr/S-informer/Presse-Communiques-Points-presse/Mise-en-garde-sur-l-utilisation-de-la-pioglitazone-en-traitement-chronique-chez-les-patients-diabetiques-Actos-R-Competact-R-Communique. Accessed 17 Dec 2015.
Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012;55(7):1953–62.
ANSM. Suspension de l’utilisation des médicaments contenant de la pioglitazone (Actos®, Competact®) - Communiqué du 09/06/2011. http://ansm.sante.fr/S-informer/Presse-Communiques-Points-presse/Suspension-de-l-utilisation-des-medicaments-contenant-de-la-pioglitazone-Actos-R-Competact-R-Communique. Accessed 17 Dec 2015.
ANSM. Etat des lieux de la consommation des benzodiazépines en France. 2013. http://ansm.sante.fr/var/ansm_site/storage/original/application/3e06749ae5a50cb7ae80fb655dee103a.pdf. Accessed 5 May 2017.
ANSM. Tétrazépam (Myolastan et génériques): des effets indésirables cutanés parfois graves sont susceptibles de remettre en cause le rapport bénéfice/risque de ces spécialités - Point d’information du 11/01/2013. http://ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Tetrazepam-Myolastan-et-generiques-des-effets-indesirables-cutanes-parfois-graves-sont-susceptibles-de-remettre-en-cause-le-rapport-benefice-risque-de-ces-specialites-Point-d-information. Accessed 17 Dec 2015.
ANSM. Spécialités à base de tétrazépam : suspension des autorisations de mise sur le marché à compter du 8 juillet 2013 - Lettre aux professionnels de santé du 02/07/2013. http://ansm.sante.fr/S-informer/Informations-de-securite-Retraits-de-lots-et-de-produits/Retrait-de-tous-les-lots-des-specialites-a-base-de-tetrazepam. Accessed 17 Dec 2015.
INSEE (Institut National de la Statistique et des Etudes Economiques). http://www.insee.fr/fr/themes/detail.asp?ref_id=estim-pop®_id=99. Accessed 6 Jul 2016.
ANSM. Compte-rendu de la commission nationale de pharmacovigilance du 22/05/2012. Suivi national de pharmacovigilance du tramadol. http://ansm.sante.fr/var/ansm_site/storage/original/application/1419194e800742d8db30bc1972415586.pdf. Accessed 17 Dec 2015.
Abadie D, Durrieu G, Roussin A, Montastruc JL. “Serious” adverse drug reactions with tramadol: a 2010–2011 pharmacovigilance survey in France [in French]. Therapie. 2013;68(2):77–84.
Pariente A, Grégoire F, Fourrier-Reglat A, Haramburu F, Moore N. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: the notoriety bias. Drug Saf. 2007;30(10):891–8.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–96.
Weber J. Epidemiology of adverse reactions to nonsteroidal anti-inflammatory drugs. Adv Inflamm Res. 1984;6:1–7.
Acknowledgements
The authors would like to acknowledge ANSM (Agence Nationale de Sécurité du Médicament et des produits de santé).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
HEADS (HEAlth Determinants in Societies) is funded by IdEx (Initiative d’excellence) of Bordeaux University.
Conflict of interest
Cécile Pageot, Julien Bezin, Andy Smith, Mickael Arnaud, Francesco Salvo, Françoise Haramburu, Bernard Bégaud and Antoine Pariente have no conflicts of interest that are directly relevant to the content of this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pageot, C., Bezin, J., Smith, A. et al. Impact of Medicine Withdrawal on Reporting of Adverse Events Involving Therapeutic Alternatives: A Study from the French Spontaneous Reporting Database. Drug Saf 40, 1099–1107 (2017). https://doi.org/10.1007/s40264-017-0561-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-017-0561-y