Abstract
Introduction
Large databases of clinician reported (e.g., allergy repositories) and patient reported (e.g., social media) adverse drug reactions (ADRs) exist; however, whether patients and clinicians report the same concerns is not clear.
Objectives
Our objective was to compare electronic health record data and social media data to better understand differences and similarities between clinician-reported ADRs and patients’ concerns regarding aspirin and atorvastatin.
Methods
This pilot study explored a large repository of electronic health record data and social media data for clinician-reported ADRs and patients concerns for two common medications: aspirin (n = 31,817 ADRs accessible in clinical data; n = 19,186 potential ADRs accessible in social media data) and atorvastatin (n = 15,047 ADRs accessible in clinical data; n = 23,408 potential ADRs accessible in social media data).
Results
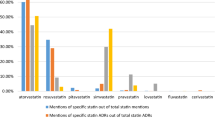
We found that the most frequently reported ADRs matched the most frequent patients’ concerns. However, several less frequently reported reactions were more prevalent on social media (i.e., aspirin-induced hypoglycemia was discussed only on social media). Overall, we found a relatively strong positive and statistically significant correlation between the frequency ranking of reactions and patients’ concerns for atorvastatin (Pearson’s r = 0.61, p < 0.001) but not for aspirin (Pearson’s r = 0.1, p = 0.69).
Conclusion
Future studies should develop further natural language methods for a more detailed data analysis (i.e., identifying causality and temporal aspects in the social media data).


Similar content being viewed by others
References
Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother. 2008;42(7):1017–25.
Ahmad SR. Adverse drug event monitoring at the Food and Drug Administration. J Gen Intern Med. 2003;18(1):57–60.
World Health Organization. Pharmacovigilance [online]. Available from: http://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/.
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001.
Rozenblum R, Bates DW. Patient-centred healthcare, social media and the internet: the perfect storm? BMJ Qual Saf. 2013;22(3):183–6.
Aramaki E, Miura Y, Tonoike M, Ohkuma T, Masuichi H, Waki K, et al. Extraction of adverse drug effects from clinical records. Stud Health Technol Inform. 2010;160(Pt 1):739–43.
Sampathkumar H, Chen X, Luo B. Mining adverse drug reactions from online healthcare forums using hidden Markov model. BMC Med Inform Decis Mak. 2014;14(1):91.
Lardon J, Abdellaoui R, Bellet F, Asfari H, Souvignet J, Texier N, et al. Adverse drug reaction identification and extraction in social media: a scoping review. J Med Internet Res. 2015;17(7):e171.
Sarker A, Nikfarjam A, O’Connor K, Ginn R, Gonzalez G, Upadhaya T, et al. Utilizing social media data for pharmacovigilance: a review. J Biomed Inform. 2015;54:202–12.
Leaman R, Wojtulewicz L, Sullivan R, Skariah A, Yang J, Gonzalez G. Towards internet-age pharmacovigilance: extracting adverse drug reactions from user posts to health-related social networks. Proc Work BioNLP. 2010;1:57–62.
Kuperman GJ, Marston E, Paterno M, Rogala J, Plaks N, Hanson C, et al. Creating an enterprise-wide allergy repository at Partners HealthCare System. AMIA Annu Symp Proc. 2003:376–80.
Topaz M, Seger D, Slight SP, Goss F, Lai K, Wickner PG, et al. Rising drug allergy alert overrides in electronic health records: an observational retrospective study of a decade of experience. J Am Med Inform Assoc. 2015. doi:10.1093/jamia/ocv143.
Topaz M, Seger DL, Lai K, Wickner PG, Goss F, Dhopeshwarkar N, et al. High override rate for opioid drug-allergy interaction alerts: current trends and recommendations for future. Stud Health Technol Inform. 2015;216:242–6.
Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255–9.
Ltd. M. Microsoft® SQL Server® 2008 Management Studio Express from Official Microsoft Download Center. 2008. Available from: http://www.microsoft.com/en-us/download/details.aspx?id=7593.
Bodenreider O. The Unified Medical Language System (UMLS): integrating biomedical terminology. Nucleic Acids Res [Internet]. 2004;32(90001):D267–70.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. About MedDRA. 2015. Available from: http://www.meddra.org/.
Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–73.
Reye’s Syndrome Information Page: National Institute of Neurological Disorders and Stroke (NINDS). Available from: http://www.ninds.nih.gov/disorders/reyes_syndrome/reyes_syndrome.htm.
StataCorp. STATA 11. College Station, TX; 2009.
Iwamoto J, Saito Y, Honda A, Matsuzaki Y. Clinical features of gastroduodenal injury associated with long-term low-dose aspirin therapy. World J Gastroenterol. 2013;19(11):1673–82.
Jolly SS, Pogue J, Haladyn K, Peters RJG, Fox KAA, Avezum A, et al. Effects of aspirin dose on ischaemic events and bleeding after percutaneous coronary intervention: insights from the PCI-CURE study. Eur Heart J. 2009;30(8):900–7.
Ayuso P, Blanca-López N, Doña I, Torres MJ, Guéant-Rodríguez RM, Canto G, et al. Advanced phenotyping in hypersensitivity drug reactions to NSAIDs. Clin Exp Allergy. 2013;43(10):1097–109.
Cazals Y. Auditory sensori-neural alterations induced by salicylate. Prog Neurobiol. 2000;62(6):583–631.
Miller RR. Deafness due to plain and long-acting aspirin tablets. J Clin Pharmacol. 1978;18(10):468–71.
Vierhapper H, Bratusch-Marrain P, Waldhäusl W, Nowotny P, Panzer S. Increased secretion of insulin but unchanged secretion of growth hormone in hyperglycaemic type II diabetics treated with acetyl-salicylic acid. Clin Endocrinol (Oxf). 1983;18(6):613–9.
Parke-Davis. Product information: LIPITOR(R) oral tablets, atorvastatin calcium oral tablets. 2012.
Tatley M, Savage R. Psychiatric adverse reactions with statins, fibrates and ezetimibe: implications for the use of lipid-lowering agents. Drug Saf. 2007;30(3):195–201.
Wagstaff LR, Mitton MW, Arvik BM, Doraiswamy PM. Statin-associated memory loss: analysis of 60 case reports and review of the literature. Pharmacotherapy. 2003;23(7):871–80.
Silverberg C. Atorvastatin-induced polyneuropathy. Ann Intern Med. 2003;139(9):792–3.
Food and Drug Administration . FDA Adverse Event Reporting System (FAERS). 2015. Available from: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/.
Acknowledgments
The authors thank Treato Ltd. for their kind assistance and collaboration on this study (no financial support was received from the company).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Agency for Healthcare Research and Quality (AHRQ), Grant #1R01HS022728-01.
Conflict of interest
Maxim Topaz, Kenneth Lai, Neil Dhopeshwarkar, Diane Seger, Ronen Rozenblum, Foster Goss, and Li Zhou report no conflicts of interest. Roee Sa’adon is a current employee of Treato Ltd., and he was involved in data cleaning and transfers but not in other data analysis.
Rights and permissions
About this article
Cite this article
Topaz, M., Lai, K., Dhopeshwarkar, N. et al. Clinicians’ Reports in Electronic Health Records Versus Patients’ Concerns in Social Media: A Pilot Study of Adverse Drug Reactions of Aspirin and Atorvastatin. Drug Saf 39, 241–250 (2016). https://doi.org/10.1007/s40264-015-0381-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-015-0381-x