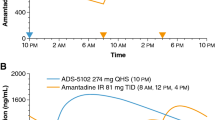
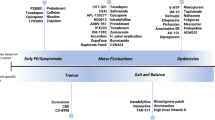
Abstract
The best practice for the initiation of symptomatic motor treatment for Parkinson’s disease is an ongoing topic of debate. Fueled by interpretation of the results of the LEAP and MED Parkinson’s disease studies, many practitioners opt for early initiation of levodopa formulations, avoiding dopamine agonists to circumvent potential deleterious side effects, namely impulse control disorder. Compared with levodopa, monoamine oxidase inhibitors may lack necessary potency. Ignored in this academic debate is another therapeutic option for patients with Parkinson’s disease requiring treatment initiation: amantadine. Amantadine was first reported effective in the treatment of Parkinson’s disease in 1969 and several studies were published in the 1970s supporting its efficacy. Currently, amantadine is mainly utilized as an add-on therapy to mitigate levodopa-related dyskinesia and, more recently, new long-acting amantadine formulations have been developed, with new indications to treat motor fluctuations. Amantadine has not been reported to cause dyskinesia and is rarely implicated in impulse control disorder.
Similar content being viewed by others
References
de Bie RMA, Clarke CE, Espay AJ, Fox SH, Lang AE. Initiation of pharmacological therapy in Parkinson’s disease: when, why, and how. Lancet Neurol. 2020;19:452–61.
Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C, Movement Disorder Society Evidence-Based Medicine Committee. International Parkinson and Movement Disorder Society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Move Disord. 2018;33:1248–66.
Hubsher G, Haider M, Okun MS. Amantadine: the journey from fighting flu to treating Parkinson disease. Neurology. 2012;78:1096–9.
Schwab RS, England AC Jr, Poskancer DC, Young RR. Amantadine in the treatment of Parkinson’s disease. JAMA. 1969;208:1168–70.
Fahn S, Isgreen WP. Long-term evaluation of amantadine and levodopa combination in parkinsonism by double-blind crossover analyses. Neurology. 1975;25:695–700.
Aoki F, Sitar D. Clinical pharmacokinetics of amantadine hydrochloride. Clin Pharmacokinet. 1988;14:35–51.
Farnebo LO, Fuxe K, Goldstein M, Hamberger B, Ungerstedt U. Dopamine and noradrenaline releasing action of amantadine in the central and peripheral nervous system: a possible mode of action in Parkinson’s disease. Eur J Pharmacol. 1971;16:27–38.
Mizoguchi K, Yokoo H, Yoshida M, et al. Amantadine increases the extracellular dopamine levels in the striatum by re-uptake inhibition and by N-methyl-D-aspartate antagonism. Brain Res. 1994;662:255–8.
Volonté MA, Moresco RM, Messa C, et al. A PET study with [11-C]raclopride in Parkinson’s disease: preliminary results on the effect of amantadine on the dopaminergic system. Neurol Sci. 2001;22:107–8.
Takahashi T, Yamashita H, Zhang YX, Nakamura S. Inhibitory effect of MK-801 on amantadine-induced dopamine release in the rat striatum. Brain Res Bull. 1996;41:363–7. https://doi.org/10.1016/s0361-9230(96)00211-0.
Gocovri (prescribers information). gocovrihcp.com. Accessed 16 Feb 2021.
Butzer JF, Silver DE, Sans AL. Amantadine in Parkinson’s disease: a double-blind, placebo-controlled, crossover study with long-term follow-up. Neurology. 1975;25:603–6.
Parkes JD, Baxter RC, Marsden CD, Rees JE. Comparative trial of benzhexol, amantadine, and levodopa in the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1974;37:422–6.
Cox B, Danta G, Schnieden H, Yuill GM. Interactions of L-dopa and amantadine in patients with Parkinsonism. J Neurol Neurosurg Psychiatry. 1973;36:354–61.
Mawdsley C, Williams IR, Pullar IA, Davidson DL, Kinlock NE. Treatment of parkinsonism by amantadine and levodopa. Clin Pharmacol Ther. 1972;13:575–83.
Fieschi C, Nardini M, Casacchia M, Tedone ME. Amantadine versus L-2 dopa and amantadine plus L-dopa. Lancet. 1970;2:154–5.
Parkes JD, Baxter RC, Curzon G, et al. Treatment of Parkinson’s disease with amantadine and levodopa: a one-year study. Lancet. 1971;1:1083–6.
Vale S, Espejel MA. Amantadine for dyskinesia tarda. N Engl J Med. 1971;284:673.
Crane G. More on amantadine for dyskinesia. N Engl J Med. 1971;285:1150–1.
Verhagen Metman L, Del Dotto P, van den Munckhof P, et al. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology. 1998;50:1323–6.
Verhagen Metman LV, Del Dotto P, LePoole K, Konitsiotis S, Fang J, Chase TN. Amantadine for levodopa-induced dyskinesias: a 1-year follow-up study. Arch Neurol. 1999;56:1383–6.
Snow BJ, Macdonald L, Mcauley D, Wallis W. The effect of amantadine on levodopa-induced dyskinesias in Parkinson’s disease: a double-blind, placebo-controlled study. Clin Neuropharmacol. 2000;23:82–5.
Luginger E, Wenning GK, Bosch S, Poewe W. Beneficial effects of amantadine on L-dopa-induced dyskinesias in Parkinson’s disease. Mov Disord. 2000;15:873–8.
Sawada H, Oeda T, Kuno S, Nomoto M, Yamamoto K, Yamamoto M, et al. Amantadine Study Group. Amantadine for dyskinesias in Parkinson’s disease: a randomized controlled trial. PLoS ONE. 2010;5:e15298. https://doi.org/10.1371/journal.pone.0015298.
Elmer L, Juncos J, Singer C, Truong D, Criswell S, Parashos S, Felt L, et al. Pooled analyses of phase III studies of ADS-5102 (Amantadine) extended-release capsules for dyskinesia in Parkinson’s disease. CNS Drugs. 2018;32:387–98.
Osmolex (prescribers information) label. fda.gov. Accessed 16 Feb 2021.
Symmetrel (prescribers information) label. fda.gov. Accessed 16 Feb 2021.
deVries T, Dentiste A, Handiwala L, Jacobs D. Bioavailability and pharmacokinetics of once-daily amantadine extended-release tablets in healthy volunteers: results from three randomized, crossover, open-label, phase 1 studies. Neurol Ther. 2019;8:449–60.
Blanchard DL. Amantadine caused corneal edema. Cornea. 1990;9:181.
Weintraub D, Sohr M, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Amantadine use associated with impulse control disorders in Parkinson disease in cross-sectional study. Ann Neurol. 2010;68:963–8.
Garcia-Ruiz PJ, Martinez Castrillo JC, Alonso-Canovas A, Herranz Barcenas A, Vela L, Sanchez Alonso P, et al. Impulse control disorder in patients with Parkinson’s disease under dopamine agonist therapy: a multicentre study. J Neurol Neurosurg Psychiatry. 2014;85:840–4. https://doi.org/10.1136/jnnp-2013-306787.
Weintraub D. Impulse control disorders in Parkinson’s disease: prevalence and possible risk factors. Parkinsonism Relat Disord. 2009;3:S110–3. https://doi.org/10.1016/S1353-8020(09)70794-1.
Redenšek S, Jenko Bizjan B, Trošt M, Dolžan V. Clinical and clinical-pharmacogenetic models for prediction of the most common psychiatric complications due to dopaminergic treatment in Parkinson’s disease. Int J Neuropsychopharmacol. 2020;23:496–504. https://doi.org/10.1093/ijnp/pyaa028.
Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–58.
Quinn N, Critchley P, Marsden CD. Young onset Parkinson’s disease. Mov Disord. 1987;2:73–91. https://doi.org/10.1002/mds.870020201.
Ku S, Glass GA. Age of Parkinson’s disease onset as a predictor for the development of dyskinesia. Mov Disord. 2010;25:1177–82.
Brüggemann N, Klein C. Parkin type of early-onset Parkinson disease. 2001 Apr 17 [updated 2020 Apr 23]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews®. Seattle (WA): University of Washington; 1993-2021. https://www.ncbi.nlm.nih.gov/books/NBK1478/. Accessed 16 Feb 2021.
Pahwa R, Tanner CM, Hauser RA, Isaacson SH, Nausieda PA, Truong DD, et al. ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson disease (EASE LID Study): a randomized clinical trial. JAMA Neurol. 2017;74:941–9. https://doi.org/10.1001/jamaneurol.2017.0943.
Oertel W, Eggert K, Pahwa R, Tanner CM, Hauser RA, Trenkwalder C, et al. Randomized, placebo-controlled trial of ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson’s disease (EASE LID 3). Mov Disord. 2017;32:1701–9. https://doi.org/10.1002/mds.27131.
Perez-Lloret S, Rey M, Fabre N, Ory F, Spampinato U, Brefel-Courbon C, et al. Prevalence and pharmacological factors associated with impulse-control disorder symptoms in patients with Parkinson disease. Clin Neuropharmacol. 2012;35:261–5. https://doi.org/10.1097/WNF.0b013e31826e6e6d.
Thomas A, Bonanni L, Gambi F, Di Iorio A, Onofrj M. Pathological gambling in Parkinson disease is reduced by amantadine. Ann Neurol. 2010;68:400–4. https://doi.org/10.1002/ana.22029.
Sharma VD, Lyons KE, Pahwa R. Amantadine extended-release capsules for levodopa-induced dyskinesia in patients with Parkinson’s disease. Ther Clin Risk Manag. 2018;14:665–73. https://doi.org/10.2147/TCRM.S144481.
Constantinescu R. Update on the use of pramipexole in the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2008;4:337–52. https://doi.org/10.2147/ndt.s2325.
Requip (prescribers information). https://www.accessdata.fda.gov/. Accessed 16 Jul 2021.
Pollack AE, Turgeon SM, Fink JS. Apomorphine priming alters the response of striatal outflow pathways to D2 agonist stimulation in 6-hydroxydopamine-lesioned rats. Neuroscience. 1997;79:79–93. https://doi.org/10.1016/s0306-4522(96)00681-1.
Rasagiline (prescribers information). https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/201971Orig1s000lbl.pdf. Accessed 16 Jul 2021.
Xadago (prescribers information). https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/207145lbl.pdf. Accessed 16 Feb 2021.
Nourianz (prescribers information). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/022075Orig1s000ClinPharmR.pdf. Accessed 16 Jul 2021.
Barbosa P, Lees AJ, Magee C, Djamshidian A, Warner TT. A retrospective evaluation of the frequency of impulsive compulsive behaviors in Parkinson’s disease patients treated with continuous waking day apomorphine pumps. Mov Disord Clin Pract. 2016;4:323–8. https://doi.org/10.1002/mdc3.12416.
Grassi G, Albani G, Terenzi F, et al. New pharmacological and neuromodulation approaches for impulsive-compulsive behaviors in Parkinson’s disease. Neurol Sci. 2021;42:2673–82. https://doi.org/10.1007/s10072-021-05237-8.
Warren N, O’Gorman C, Lehn A, Siskind D. Dopamine dysregulation syndrome in Parkinson’s disease: a systematic review of published cases. J Neurol Neurosurg Psychiatry. 2017;88:1060–4. https://doi.org/10.1136/jnnp-2017-315985.
Ramirez-Zamora A, Gee L, Boyd J, Biller J. Treatment of impulse control disorders in Parkinson’s disease: practical considerations and future directions. Expert Rev Neurother. 2016;16:389–99. https://doi.org/10.1586/14737175.2016.115810.3.
Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351:2498–508.
Verschuur CVM, Suwijn SR, Boel JA, Post B, Bloem BR, van Hilten JJ, et al. Randomized delayed-start trial of levodopa in Parkinson’s disease. N Engl J Med. 2019;380:315–24. https://doi.org/10.1056/NEJMoa1809983.
Isaacson SH, Fahn S, Pahwa R, Tanner CM, Espay AJ, Trenkwalder C, et al. Parkinson’s patients with dyskinesia switched from immediate release amantadine to open-label ADS-5102. Mov Disord Clin Pract. 2018;5:183–90. https://doi.org/10.1002/mdc3.12595.
Hauser RA, Walsh RR, Pahwa R, Chernick D, Formella AE. Amantadine ER (Gocovri®) significantly increases ON time without any dyskinesia: pooled analyses from pivotal trials in Parkinson’s disease. Front Neurol. 2021;12:645706.
Tanner CM, Pahwa R, Hauser RA, et al. EASE LID 2: a 2-year open-label trial of Gocovri (amantadine) extended release for dyskinesia in Parkinson’s disease. J Parkinsons Dis. 2020;10:543–58.
Agbo F, Isaacson SH, Gil R, et al. Pharmacokinetics and comparative bioavailability of apomorphine sublingual film and subcutaneous apomorphine formulations in patients with parkinson’s disease and “OFF” episodes: results of a randomized, three-way crossover, open-label study. Neurol Ther. 2021. https://doi.org/10.1007/s40120-021-00251-6.
Thijssen E, den Heijer J, Puibert D, Lei M, Hasegawa D, Keum K, Mochel K, Roset P, van Brummelen E, Naranda T, Groeneveld G (2021) Randomized, placebo-controlled study investigating the safety, pharmacokinetics and efficacy of inhaled apomorphine in Parkinson’s disease patients [abstract]. Mov Disord. 2020;35(suppl 1). https://www.mdsabstracts.org/abstract/randomized-placebo-controlled-study-investigating-the-safetypharmacokinetics-and-efficacy-of-inhaled-apomorphine-in-parkinsons-disease-patients/. Accessed 25 Sept 2021.
Shpiner DS, Bette S, Di Luca DG, Margolesky J. CVT-301 for the treatment of Parkinson’s disease. Expert Rev Neurother. 2019;19(7):603–11. https://doi.org/10.1080/14737175.2019.1621748.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There was no funding source associated with this manuscript.
Conflicts of interest/competing interests
Sarah Marmol has no conflicts of interest to declare. Matthew Feldman has no conflicts of interest to declare. Carlos Singer has received grants from Pharma2B, Sunovion, Adamas, Revance, and Amneal, and honoraria from Abbvie and Mitsubishi. Jason Margolesky has no conflicts of interest to declare.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
SM performed the literature review, contributed significantly to the revised manuscript, and was co first author with MF. MF performed the literature review, contributed significantly to the revised manuscript, and was co first author with SM. CS conceptualized, designed, and provided revision to the manuscript. JM conceptualized, designed, and drafted the manuscript and performed the literature review. All authors approve the final version of the manuscript for submission and publication, and agree to be accountable for the work presented.
Rights and permissions
About this article
Cite this article
Marmol, S., Feldman, M., Singer, C. et al. Amantadine Revisited: A Contender for Initial Treatment in Parkinson’s Disease?. CNS Drugs 35, 1141–1152 (2021). https://doi.org/10.1007/s40263-021-00862-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-021-00862-5