Abstract
Introduction
Natalizumab has proved to be more effective than fingolimod in reducing disease activity in relapsing-remitting multiple sclerosis (RRMS). Whether this association is universal for all patient groups remains to be determined.
Objective
The aim of this study was to compare the relative effectiveness of natalizumab and fingolimod in RRMS subgroups defined by the baseline demographic and clinical characteristics of interest.
Methods
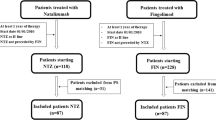
Patients with RRMS who were given natalizumab or fingolimod were identified in a merged cohort from three international registries. Efficacy outcomes were compared across subgroups based on patients’ sex, age, disease duration, Expanded Disability Status Scale (EDSS) score, and disease and magnetic resonance imaging (MRI) activity 12 months prior to treatment initiation. Study endpoints were number of relapses (analyzed with weighted negative binomial generalized linear model) and 6-month confirmed disability worsening and improvement events (weighted Cox proportional hazards model), recorded during study therapy. Each patient was weighted using inverse probability of treatment weighting based on propensity score.
Results
A total of 5148 patients (natalizumab 1989; fingolimod 3159) were included, with a mean ± standard deviation age at baseline of 38 ± 10 years, and the majority (72%) were women. The median on-treatment follow-up was 25 (quartiles 15–41) months. Natalizumab was associated with fewer relapses than fingolimod (incidence rate ratio [IRR]; 95% confidence interval [CI]) in women (0.76; 0.65–0.88); in those aged ≤ 38 years (0.64; 0.54–0.76); in those with disease duration ≤ 7 years (0.63; 0.53–0.76); in those with EDSS score < 4 (0.75; 0.64–0.88), < 6 (0.80; 0.70–0.91), and ≥ 6 (0.52; 0.31–0.86); and in patients with pre-baseline relapses (0.74; 0.64–0.86). A higher probability of confirmed disability improvement on natalizumab versus fingolimod (hazard ratio [HR]; 95% CI) was observed among women (1.36; 1.10–1.66); those aged > 38 years (1.34; 1.04–1.73); those with disease duration > 7 years (1.33; 1.01–1.74); those with EDSS score < 6 (1.21; 1.01–1.46) and ≥ 6 (1.93; 1.11–3.34); and patients with no new MRI lesion (1.73; 1.19–2.51).
Conclusions
Overall, in women, younger patients, those with shorter disease durations, and patients with pre-treatment relapses, natalizumab was associated with a lower frequency of multiple sclerosis relapses than fingolimod. It was also associated with an increased chance of recovery from disability among most patients, particularly women and those with no recent MRI activity.




Similar content being viewed by others
References
Kappos L, Radue E-W, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401.
Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910.
Barbin L, Rousseau C, Jousset N, et al. Comparative efficacy of fingolimod vs natalizumab: a French multicenter observational study. Neurology. 2016;86(8):771–8.
Kalincik T, Horakova D, Spelman T, et al. Switch to natalizumab versus fingolimod in active relapsing–remitting multiple sclerosis. Ann Neurol. 2015;77(3):425–35.
Lorscheider J, Benkert P, Lienert C, et al. Comparative analysis of natalizumab versus fingolimod as second-line treatment in relapsing–remitting multiple sclerosis. Mult Scler J. 2018;24(6):777–85.
Prosperini L, Saccà F, Cordioli C, et al. Real-world effectiveness of natalizumab and fingolimod compared with self-injectable drugs in non-responders and in treatment-naïve patients with multiple sclerosis. J Neurol. 2017;264(2):284–94.
Kalincik T, Manouchehrinia A, Sobisek L, et al. Towards personalized therapy for multiple sclerosis: prediction of individual treatment response. Brain. 2017;140(9):2426–43.
Hutchinson M, Kappos L, Calabresi PA, et al. The efficacy of natalizumab in patients with relapsing multiple sclerosis: subgroup analyses of AFFIRM and SENTINEL. J Neurol. 2009;256(3):405–15.
Devonshire V, Havrdova E, Radue EW, et al. Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol. 2012;11(5):420–8.
Koch-Henriksen N, Magyari M, Sellebjerg F, et al. A comparison of multiple sclerosis clinical disease activity between patients treated with natalizumab and fingolimod. Mult Scler J. 2017;23(2):234–41.
Andersen JB, Sharmin S, Lefort M, et al. The effectiveness of natalizumab vs fingolimod–A comparison of international registry studies. Multiple Scler Relat Disord. 2021;53:103012.
Kalincik T, Butzkueven H. The MSBase registry: Informing clinical practice. Mult Scler J. 2019;25(14):1828–34.
Vukusic S, Casey R, Rollot F, et al. Observatoire Français de la Sclérose en Plaques (OFSEP): a unique multimodal nationwide MS registry in France. Mult Scler J. 2020;26(1):118–22.
Koch-Henriksen N, Magyari M, Laursen B. Registers of multiple sclerosis in Denmark. Acta Neurol Scand. 2015;132:4–10.
Kalincik T, Kuhle J, Pucci E, et al. Data quality evaluation for observational multiple sclerosis registries. Mult Scler J. 2017;23(5):647–55.
Confavreux C, Compston D, Hommes O, et al. EDMUS, a European database for multiple sclerosis. J Neurol Neurosurg Psychiatry. 1992;55(8):671–6.
Kalincik T, Cutter G, Spelman T, et al. Defining reliable disability outcomes in multiple sclerosis. Brain. 2015;138(11):3287–98.
Kappos L, Butzkueven H, Wiendl H, et al. Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving versus a fixed reference value in a prospective cohort study. Mult Scler J. 2018;24(7):963–73.
Austin PC, Stuart EA. The performance of inverse probability of treatment weighting and full matching on the propensity score in the presence of model misspecification when estimating the effect of treatment on survival outcomes. Stat Methods Med Res. 2017;26(4):1654–70.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2018. https://www.R-project.org/.
Sormani MP, Bruzzi P. Reporting of subgroup analyses from clinical trials. Lancet Neurol. 2012;11(9):747.
Kalincik T. Effectiveness of oral multiple sclerosis therapies in clinical context. Neurology. 2019;92:737–8.
Tremlett H, Zhao Y, Joseph J, et al. Relapses in multiple sclerosis are age-and time-dependent. J Neurol Neurosurg Psychiatry. 2008;79(12):1368–74.
Kalincik T, Vivek V, Jokubaitis V, et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain. 2013;136(12):3609–17.
Bove R, McHenry A, Hellwig K, et al. Multiple sclerosis in men: management considerations. J Neurol. 2016;263(7):1263–73.
Ribbons KA, McElduff P, Boz C, et al. Male sex is independently associated with faster disability accumulation in relapse-onset MS but not in primary progressive MS. PLoS ONE. 2015;10(6):e0122686.
Kunchok A, Lechner-Scott J, Granella F, et al. Prediction of on-treatment disability worsening in RRMS with the MAGNIMS score. Mult Scler J. 2021;27(5):695–705.
Putzki N, Yaldizli Ö, Bühler R, et al. Natalizumab reduces clinical and MRI activity in multiple sclerosis patients with high disease activity: results from a multicenter study in Switzerland. Eur Neurol. 2010;63(2):101–6.
Uher T, Schaedelin S, Srpova B, et al. Monitoring of radiologic disease activity by serum neurofilaments in MS. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e714.
Acknowledgements
The MSBase study contributors are listed in electronic supplementary material (ESM)-1. MSBase thanks Sara Eichau (Hospital Universitario Virgen Macarena, Sevilla, Spain), Vahid Shaygannejad (Isfahan University of Medical Sciences, Isfahan, Iran), and Rana Karabudak (Hacettepe University, Ankara, Turkey) for providing access to their data. The OFSEP investigators (steering committee, followed by investigators in descending order of number of patients included in the cohort) are listed in ESM 2. The Danish Multiple Sclerosis Registry thank the following investigators who participated in data acquisition and for providing clinical information and notification of the Danish Multiple Sclerosis Treatment Register: Alex Heick, Lars Kristian Storr, Mads Ravnborg, Matthias Kant, Nasrin Asgari, Jens Arentsen, Thor Petersen, Bjarne Sivertsen, Helle Hvilsted Nielsen, Georgi Sirakov, Allan Pedersen, and Mette Kirstine Christensen. They also thank the secretariat of the Clinical Quality Databases under Danish Regions for allowing them to use data from the Danish Multiple Sclerosis Treatment Register for this study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
The Clinical Outcomes Research unit at the University of Melbourne received funding from the National Health and Medical Research Council (Grant numbers 1140766, 1129789, and 1157717) to support this study. The MSBase Foundation is a not-for-profit organization that receives support from Merck, Biogen, Novartis, Roche, Bayer Schering, Sanofi Genzyme, and Teva Pharmaeutical Industries. OFSEP was supported by a grant provided by the French State and handled by the "Agence Nationale de la Recherche," within the framework of the "Investments for the Future" program, under the reference ANR-10-COHO-002, by the Eugène Devic EDMUS Foundation against multiple sclerosis and by the ARSEP Foundation. The Danish Multiple Sclerosis Registry did not receive any funding to collaborate in this study.
Conflicts of Interest
Authors of the MSBase registry: The following authors have received speaker honoraria, advisory board or steering committee fees, research support, and/or conference travel support from Actelion (EKH), Almirall (GI, FP, MT), Bayer (RA, FP, AL, MT, CB, MT, MS, JLS, BVW, TC, DS), BioCSL (KB, TK), Biogen (DH, EKH, RA, GI, FP, AL, PG, FGM, MG, PD, CB, MT, MS, JLS, PS, DF, FG, JP, BVW, TC, HB, TK), Canadian Multiple sclerosis society (PG, PD), Canadian Institutes of Health Research (MG, PD), Celgene (EKH, FP, TK), Czech Ministry of Education (DH, EKH), Fondazione Italiana Sclerosi Multipla (FP, AL), Grifols (KB), Genzyme-Sanofi (DH, EKH, RA, GI, FP, AL, MT, PG, FGM, MG, PD, CB, MT, MS, JLS, PS, DF, FG, JP, BVW, TC, DS, HB, TK), GSK (RA), Merck / EMD (DH, EKH, RA, GI, FP, AL, MT, PG, MG, PD, CB, MT, MS, JLS, PS, DF, FG, KB, BVW, TC, DS, HB, TK), Mitsubishi (FGM), Ministero Italiano della Universit e della Ricerca Scientifica (FP), Mylan (FP, AL), Novartis (DH, EKH, RA, GI, FP, AL, MT, PG, FGM, MG, PD, CB, MT, MS, JLS, PS, DF, FG, JP, KB, BVW, TC, DS, HB, TK), ONO Pharmaceuticals (FGM), Roche (DH, EKH, RA, GI, FP, AL, MT, CB, FG, KB, BVW, TC, TK), Teva (DH, EKH, GI, FP, AL, MT, PG, FGM, MG, PD, CB, MT, JLS, PS, DF, JP, KB, BVW, TC, DS, TK), WebMD Global (TK). Authors of the French Multiple Sclerosis registry have received speaker honoraria, advisory board or steering committee fees, independent data monitoring committees fee, consultancy or lecturing fees, research support, unconditional PhD donation, and/or conference travel support from or served as principal investigators in clinical trials for Actelion (PC, ET), Ad Scientiam (EM), Akcea (JPC), Alnylam (JPC), Almirall (OH), Bayer (GE, HZ, OH), Biogen (GE, JC, AR, JDS, EM, HZ, PL, GD, TM, EB, PC, JP, BS, ET, OH, BB, OC, AMo, JPC, AMa, IP, NM, CL, DAL, SV, AW), Celgene (ET, DAL), CSL-Behring (JPC), FHU Imminent (HZ), Geneuro (SV), Genzyme-Sanofi (GE, JC, AR, JDS, EM, HZ, PL, GD, CLF, TM, EB, PC, JP, BS, ET, OH, BB, OC, AMo, JPC, AMa, IP, NM, CL, DAL, SV), Grifols (JPC), Laboratoire Français des Biotechnologies (JPC), LFB (GE), LFSEP (HZ), Merck / EMD (GE, JC, AR, EM, HZ, PL, GD, PC, JP, BS, ET, OH, BB, OC, AMo, JPC, AMa, NM, DAL, SV), Medday (EL, AR, TM, PC, JP, DAL, SV), Natus (JPC), Novartis (EL, GE, JC, AR, JDS, EM, HZ, PL, GD, CLF, TM, EB, PC, JP, BS, ET, OH, BB, OC, AMo, JPC, AMa, IP, NM, CL, DAL, SV), Pfizer (JPC), Pharmalliance (JPC), Roche (ML, EL, GE, JC, AR, JDS, EM, HZ, PL, GD, CLF, EB, PC, JP, BS, ET, OH, BB, OC, AMa, IP, NM, DAL, SV, AW), SNF-Floerger (JPC), Teva (GE, JC, AR, JDS, EM, HZ, PL, GD, EB, PC, JP, ET, OH, BB, AMo, JPC, AMa, SV), Académie de Médecine (HZ), Agence Nationale de la Recherche (DAL), French National Security Agency of Medicines and Health Products (EL), the EDMUS Foundation (EL), the ARSEP foundation (ML, GE, HZ, ET, DAL), PHRC Foundation (ET), Rennes University Hospital (GE). Authors of the Danish Multiple Sclerosis Registry have received speaker honoraria, advisory board or steering committee fees, independent data monitoring committees fee, consultancy fees, research support, and/or conference travel support from Almirall (JF), Bayer (HKM), Biogen (NKH, FS, CH, PVR, MBJ, JF, SB, HKM, KIS, MM), Celgene (PSS), Genzyme-Sanofi (FS, PSS, CH, PVR, MBJ, JF, SB, HKM, KIS, MM), GSK (PSS), Medday (PSS), Merck / EMD (JBA, NKH, FS, PSS, CH, PVR, MBJ, JF, SB, HKM, KIS, MM), Novartis (NKH, FS, PSS, CH, PVR, MBJ, JF, KIS, MM), Roche (FS, CH, PVR, MBJ, JF, SB, KIS, MM), Teva (NKH, FS, PSS, PVR, MBJ, JF, HKM, KIS, MM).
Ethics approval
Ethics approval was obtained from the appropriate authorities. Please see Sect. 2.1 for details.
Consent to participate
Consent was obtained from each patient included in this study. Please see Sect. 2.1 for details.
Consent for publication
Not applicable.
Availability of data and material
MSBase is a data processor and warehouses data from individual principal investigators who agree to share their datasets on a project-by-project basis. Each principal investigator will need to be approached individually for permission to access the datasets. The OFSEP data can be obtained upon request after evaluation by the OFSEP scientific committee and validation from the OFSEP steering committee. Details can be found on http://www.ofsep.org/en/data-access. Data from DMSR will be shared upon request from a qualified investigator under approval from the Danish Data Protection Agency and the board of the Danish Multiple Sclerosis Group.
Code availability
Available upon request.
Authors' contributions
SS, ML, and JBA contributed to the design of the study, conducted and interpreted the analysis, and drafted, revised, and approved the manuscript. MM, SV, HB, and TK conceptualized and designed the study, contributed to data acquisition, interpreted the results, and revised and approved the manuscript. EL, RC, and DL contributed to the design of the study, interpreted the results, and revised and approved the manuscript. DH, EKH, RA, GI, SO, FP, MO, AL, MT, PG, FGM, BY, AP, MG, PD, CB, MT, PM, MS, JLS, RT, PS, DF, FG, JP, DM, OS, KB, AVW, BVW, TC, DS, SV, MD, GE, JC, AR, JDS, EM, HZ, PL, GD, CLF, TM, EB, PC, JP, BS, OG, ET, OH, AAK, BB, OC, PC, AM, AW, JPC, AM, IP, KH, CP, NM, CL, CN, NKH, FTS, PSS, CCP, PVR, MBJ, JLF, SB, HKM, and KIS contributed substantially to data acquisition and interpretation of the analysis, and revised and approved the manuscript. SS, ML, and JBA contributed equally. MM, SV, HB, and TK contributed equally. All authors approved the final version of the manuscript for submission and publication and thus assume responsibility for the work presented in the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharmin, S., Lefort, M., Andersen, J.B. et al. Natalizumab Versus Fingolimod in Patients with Relapsing-Remitting Multiple Sclerosis: A Subgroup Analysis From Three International Cohorts. CNS Drugs 35, 1217–1232 (2021). https://doi.org/10.1007/s40263-021-00860-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-021-00860-7