Abstract
Dravet syndrome is a rare but severe epilepsy syndrome that begins in the first year of life with recurrent seizures triggered by fever that are typically prolonged and hemiclonic. The epilepsy is highly drug resistant. Although development is normal at onset, over time, most patients develop moderate-to-severe intellectual disability, behavior disorders, and a characteristic crouch gait. There is a significant mortality, predominantly owing to sudden unexpected death in epilepsy. Complete seizure control is rarely attainable. Initial therapy includes valproic acid and clobazam, but response is typically inadequate. The results of new drugs for Dravet syndrome, including stiripentol, cannabidiol, and fenfluramine, are very promising. Stiripentol was associated with a greater than 50% reduction in convulsive seizure frequency in 71% of cases, when added to valproic acid and clobazam, and also markedly reduced status epilepticus. Pharmaceutical-grade cannabidiol resulted in a median change in monthly motor seizures from baseline of − 36.5%. Fenfluramine was associated with a greater than 50% reduction in seizures of 70%, with one quarter of cases achieving near seizure freedom over the duration of the trial. These agents are generally well tolerated, with few patients discontinuing for adverse effects. There is limited evidence to date regarding improvement in cognition with these newer agents; however, a meaningful change is challenging to assess over short trial periods and requires longer follow-up studies. While current treatments focus predominantly on seizure control, newer therapies including genetic treatments and antisense oligonucleotides can target the SCN1A channelopathy, and thus, may also significantly impact the important co-morbidities associated with this syndrome.


Similar content being viewed by others
References
Dravet C, Bureau M, Oguni H, et al. Dravet syndrome (severe myoclonic epilepsy in infancy). In: Bureau M, Genton P, Dravet C, editors. Epileptic syndromes in infancy, childhood and adolescence. Paris: John Libbey Eurotext; 2012. p. 125–56.
De Liso P, Chemaly N, Laschet J, et al. Patients with Dravet syndrome in the era of stiripentol: a French cohort cross-sectional study. Epilepsy Res. 2016;125:42–6.
Losito E, Kuchenbuch M, Chemaly N, et al. Age-related “sleep/nocturnal” tonic and tonic clonic seizure clusters are underdiagnosed in patients with Dravet syndrome. Epilepsy Behav. 2017;74:33–40.
Ragona F. Cognitive development in children with Dravet syndrome. Epilepsia. 2011;52(Suppl. 2):39–43.
Nabbout R, Chemaly N, Chipaux M, et al. Encephalopathy in children with Dravet syndrome is not a pure consequence of epilepsy. Orphanet J Rare Dis. 2013;8:176.
Li BM, Liu XR, Yi YH, et al. Autism in Dravet syndrome: prevalence, features, and relationship to the clinical characteristics of epilepsy and mental retardation. Epilepsy Behav. 2011;21:291–5.
Ouss L, Leunen D, Laschet J, et al. Autism spectrum disorder and cognitive profile in children with Dravet syndrome: delineation of a specific phenotype. Epilepsia Open. 2019;4:40–53.
Skluzacek JV, Watts KP, Parsy O, Wical B, Camfield P. Dravet syndrome and parent associations: the IDEA League experience with comorbid conditions, mortality, management, adaptation, and grief. Epilepsia. 2011;52(Suppl. 2):95–101.
Villas N, Meskis MA, Goodliffe S. Dravet syndrome: characteristics, comorbidities, and caregiver concerns. Epilepsy Behav. 2017;74:81–6.
Nabbout R, Auvin S, Chiron C, et al. Development and content validation of a preliminary core set of patient- and caregiver-relevant outcomes for inclusion in a potential composite endpoint for Dravet Syndrome. Epilepsy Behav. 2018;78:232–42.
Nabbout R, Auvin S, Chiron C, et al. Perception of impact of Dravet syndrome on children and caregivers in multiple countries: looking beyond seizures. Dev Med Child Neurol. 2019;61:1229–36.
Lagae L, Brambilla I, Mingorance A, Gibson E, Battersby A. Quality of life and comorbidities associated with Dravet syndrome severity: a multinational cohort survey. Dev Med Child Neurol. 2018;60:63–72.
Rodda JM, Scheffer IE, McMahon JM, Berkovic SF, Graham HK. Progressive gait deterioration in adolescents with Dravet syndrome. Arch Neurol. 2012;69:873–8.
Genton P, Velizarova R, Dravet C. Dravet syndrome: the long-term outcome. Epilepsia. 2011;52(Suppl. 2):44–9.
Jensen MP, Brunklaus A, Dorris L, et al. The humanistic and economic burden of Dravet syndrome on caregivers and families: implications for future research. Epilepsy Behav. 2017;70:104–9.
Cooper MS, McIntosh A, Crompton DE, et al. Mortality in Dravet syndrome. Epilepsy Res. 2016;128:43–7.
Wirrell EC, Laux L, Donner E, et al. Optimizing the diagnosis and management of Dravet syndrome: recommendations from a North American Consensus Panel. Pediatr Neurol. 2017;68(18–34):e13.
Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–32.
de Lange IM, Koudijs MJ, van’t Slot R, et al. Mosaicism of de novo pathogenic SCN1A variants in epilepsy is a frequent phenomenon that correlates with variable phenotypes. Epilepsia. 2018;59:690–703.
Ceulemans B. Overall management of patients with Dravet syndrome. Dev Med Child Neurol. 2011;53(Suppl. 2):19–23.
Wilmshurst JM, Gaillard WD, Vinayan KP, et al. Summary of recommendations for the management of infantile seizures: task Force Report for the ILAE Commission of Pediatrics. Epilepsia. 2015;56:1185–97.
Thanh TN, Chiron C, Dellatolas G, et al. Long-term efficacy and tolerance of stiripentaol in severe myoclonic epilepsy of infancy (Dravet’s syndrome). Arch Pediatr. 2002;9:1120–7.
Saito Y, Oguni H, Awaya Y, Hayashi K, Osawa M. Phenytoin-induced choreoathetosis in patients with severe myoclonic epilepsy in infancy. Neuropediatrics. 2001;32:231–5.
de Lange IM, Gunning B, Sonsma ACM, et al. Influence of contraindicated medication use on cognitive outcome in Dravet syndrome and age at first afebrile seizure as a clinical predictor in SCN1A-related seizure phenotypes. Epilepsia. 2018;59:1154–65.
Snoeijen-Schouwenaars FM, Veendrick MJ, van Mierlo P, et al. Carbamazepine and oxcarbazepine in adult patients with Dravet syndrome: friend or foe? Seizure. 2015;29:114–8.
Guerrini R, Dravet C, Genton P, Belmonte A, Kaminska A, Dulac O. Lamotrigine and seizure aggravation in severe myoclonic epilepsy. Epilepsia. 1998;39:508–12.
Dalic L, Mullen SA, Roulet Perez E, Scheffer I. Lamotrigine can be beneficial in patients with Dravet syndrome. Dev Med Child Neurol. 2015;57:200–2.
Mueller A, Boor R, Coppola G, et al. Low long-term efficacy and tolerability of add-on rufinamide in patients with Dravet syndrome. Epilepsy Behav. 2011;21:282–4.
Chipaux M, Villeneuve N, Sabouraud P, et al. Unusual consequences of status epilepticus in Dravet syndrome. Seizure. 2010;19:190–4.
Dressler A, Trimmel-Schwahofer P, Reithofer E, et al. Efficacy and tolerability of the ketogenic diet in Dravet syndrome: comparison with various standard antiepileptic drug regimen. Epilepsy Res. 2015;109:81–9.
Inoue Y, Ohtsuka Y, Oguni H, et al. Stiripentol open study in Japanese patients with Dravet syndrome. Epilepsia. 2009;50:2362–8.
Redondo P, Vicente J, Espana A, Subira ML, De Felipe I, Quintanilla E. Photo-induced toxic epidermal necrolysis caused by clobazam. Br J Dermatol. 1996;135:999–1002.
Yapici AK, Fidanci MK, Kilic S, et al. Stevens-Johnson syndrome triggered by a combination of clobazam, lamotrigine and valproic acid in a 7-year-old child. Ann Burns Fire Disasters. 2014;27:121–5.
Tanabe T, Awaya Y, Matsuishi T, et al. Management of and prophylaxis against status epilepticus in children with severe myoclonic epilepsy in infancy (SMEI; Dravet syndrome): a nationwide questionnaire survey in Japan. Brain Dev. 2008;30:629–35.
Friedlander WJ. The rise and fall of bromide therapy in epilepsy. Arch Neurol. 2000;57:1782–5.
Lotte J, Haberlandt E, Neubauer B, Staudt M, Kluger GJ. Bromide in patients with SCN1A-mutations manifesting as Dravet syndrome. Neuropediatrics. 2012;43:17–21.
Oguni H, Hayashi K, Oguni M, et al. Treatment of severe myoclonic epilepsy in infants with bromide and its borderline variant. Epilepsia. 1994;35:1140–5.
Dibue-Adjei M, Fischer I, Steiger HJ, Kamp MA. Efficacy of adjunctive vagus nerve stimulation in patients with Dravet syndrome: a meta-analysis of 68 patients. Seizure. 2017;50:147–52.
Fulton SP, Van Poppel K, McGregor AL, Mudigoudar B, Wheless JW. Vagus nerve stimulation in intractable epilepsy associated with SCN1A gene abnormalities. J Child Neurol. 2017;32:494–8.
Dlouhy BJ, Miller B, Jeong A, Bertrand ME, Limbrick DD Jr, Smyth MD. Palliative epilepsy surgery in Dravet syndrome: case series and review of the literature. Childs Nerv Syst. 2016;32:1703–8.
Poisson M, Huguet F, Savattier A, Bakri-Logeais F, Narcisse G. A new type of anticonvulsant, stiripentol: pharmacological profile and neurochemical study. Arzneimittelforschung. 1984;34:199–204.
Quilichini PP, Chiron C, Ben-Ari Y, Gozlan H. Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABA-A receptor channels. Epilepsia. 2006;47:704–16.
Fisher JL. The effects of stiripentol on GABA(A) receptors. Epilepsia. 2011;52(Suppl. 2):76–8.
Grosenbaugh DK, Mott DD. Stiripentol in refractory status epilepticus. Epilepsia. 2013;54(Suppl. 6):103–5.
Tran A, Rey E, Pons G, et al. Influence of stiripentol on cytochrome P450-mediated metabolic pathways in humans: in vitro and in vivo comparison and calculation of in vivo inhibition constants. Clin Pharmacol Ther. 1997;62:490–504.
Giraud C, Treluyer JM, Rey E, et al. In vitro and in vivo inhibitory effect of stiripentol on clobazam metabolism. Drug Metab Dispos. 2006;34:608–11.
Peigne S, Rey E, Le Guern ME, et al. Reassessment of stiripentol pharmacokinetics in healthy adult volunteers. Epilepsy Res. 2014;108:909–16.
Levy RH, Loiseau P, Guyot M, Blehaut HM, Tor J, Moreland TA. Stiripentol kinetics in epilepsy: nonlinearity and interactions. Clin Pharmacol Ther. 1984;36:661–9.
Yamamoto Y, Takahashi Y, Ikeda H, Imai K, Kagawa Y, Inoue Y. Impact of CYP2C19 phenotypes on clinical efficacy of stiripentol in Japanese patients with Dravet syndrome. Ther Drug Monit. 2019;15:8. https://doi.org/10.1097/ftd.0000000000000676 (Epub ahead of print).
Perez J, Chiron C, Musial C, et al. Stiripentol: efficacy and tolerability in children with epilepsy. Epilepsia. 1999;40:1618–26.
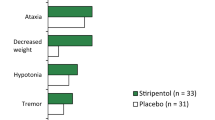
Chiron C, Marchand MC, Tran A, et al. Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome-dedicated trial. STICLO study group. Lancet. 2000;356:1638–42.
Inoue Y, Ohtsuka Y, Group STPS. Effectiveness of add-on stiripentol to clobazam and valproate in Japanese patients with Dravet syndrome: additional supportive evidence. Epilepsy Res. 2014;108:725–31.
Wirrell EC, Laux L, Franz DN, et al. Stiripentol in Dravet syndrome: results of a retrospective U.S. study. Epilepsia. 2013;54:1595–604.
Balestrini S, Sisodiya SM. Audit of use of stiripentol in adults with Dravet syndrome. Acta Neurol Scand. 2017;135:73–9.
Inoue Y, Ohtsuka Y, Group STPS. Long-term safety and efficacy of stiripentol for the treatment of Dravet syndrome: a multicenter, open-label study in Japan. Epilepsy Res. 2015;113:90–7.
Myers KA, Lightfoot P, Patil SG, Cross JH, Scheffer IE. Stiripentol efficacy and safety in Dravet syndrome: a 12-year observational study. Dev Med Child Neurol. 2018;60:574–8.
Chiron C, Helias M, Kaminska A, et al. Do children with Dravet syndrome continue to benefit from stiripentol for long through adulthood? Epilepsia. 2018;59:1705–17.
Centers for Disease Control and Prevention. Cardiac valvulopathy associated with exposure to fenfluramine or dexfenfluramine: us Department of Health and Human Services interim public health recommendations, November 1997. JAMA. 1997;278:1729–31.
Zhang Y, Kecskes A, Copmans D, et al. Pharmacological characterization of an antisense knockdown zebrafish model of Dravet syndrome: inhibition of epileptic seizures by the serotonin agonist fenfluramine. PLoS One. 2015;10:e0125898.
Sourbron J, Smolders I, de Witte P, Lagae L. Pharmacological analysis of the anti-epileptic mechanisms of fenfluramine in scn1a mutant zebrafish. Front Pharmacol. 2017;8:191.
Gastaut H. Efficacy of fenfluramine for the treatment of compulsive behavior disorders in psychotic children. Press Med. 1984;13:2024–5.
Aicardi J, Gastaut H. Treatment of self-induced photosensitive epilepsy with fenfluramine. N Engl J Med. 1985;313:1419.
Boel M, Casaer P. Add-on therapy of fenfluramine in intractable self-induced epilepsy. Neuropediatrics. 1996;27:171–3.
Casaer P, Boel M. Fenfluramine as a potential antiepileptic drug. Epilepsia. 2002;43:205–6.
Ceulemans B, Boel M, Leyssens K, et al. Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia. 2012;53:1131–9.
Schoonjans A, Paelinck BP, Marchau F, et al. Low-dose fenfluramine significantly reduces seizure frequency in Dravet syndrome: a prospective study of a new cohort of patients. Eur J Neurol. 2017;24:309–14.
Schoonjans AS, Marchau F, Paelinck BP, et al. Cardiovascular safety of low-dose fenfluramine in Dravet syndrome: a review of its benefit-risk profile in a new patient population. Curr Med Res Opin. 2017;33:1773–81.
Lagae L, Sullivan J, Cross JH, et al. ZX008 (fenfluramine) in Dravet syndrome: results of a phase 3, randomized, double-blind, placebo-controlled trial. Washington, DC: American Epilepsy Society; 2017.
Nabbout R, Auvin S, Zuberi SM, Villeneuve N, Sanchez-Carpintero R, Stephani U, et al. Fenfluramine (Fintepla®) reduces convulsive seizure frequency in Dravet syndrome patients receiving an antiepileptic drug treatment regimen containing stiripentol: a phase 3, randomized, placebo-controlled clinical trial. New Orleans: American Epilepsy Society; 2018.
Rubino C, Boyd B, Zhang L, Ismail M, Trang M, Farfel G. ZX008 (fenfluramine oral solution) as adjunctive therapy for Dravet syndrome seizures: a pharmacometric approach to quantify potential drug-drug interactions to support phase 3 dose selection. Washington, DC: American Epilepsy Society; 2017.
Lai WM, Pringsheim M, Farfel G, Galer B, Morrison G, Gammaitoni A, et al. Long-term cardiovascular safety of fenfluramine HCl (Fintepla®) in the treatment of Dravet syndrome: interim analysis of an open-label safety extension study. New Orleans: American Epilepsy Society; 2018.
Lagae L, Sullivan J, Nabbout R, Pringsheim M, Farfel G, Galer B, et al. Fenfluramine HCl (Fintepla®) provides long-term clinically meaningful reduction in seizure frequency: results of an open-label extension study. New Orleans: American Epilepsy Society; 2018.
Friedman D, Devinsky O. Cannabinoids in the treatment of eilepsy. N Engl J Med. 2015;373:1048–58.
Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15:270–8.
Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–20.
Devinsky O, Nabbout R, Miller I, et al. Long-term cannabidiol treatment in patients with Dravet syndrome: an open-label extension trial. Epilepsia. 2019;60:294–302.
Wolff M, Casse-Perrot C, Dravet C. Severe myoclonic epilepsy of infants (Dravet syndrome): natural history and neuropsychological findings. Epilepsia. 2006;47(Suppl. 2):45–8.
Coppola G, Capovilla G, Montagnini A, et al. Topiramate as add-on drug in severe myoclonic epilepsy in infancy: an Italian multicenter open trial. Epilepsy Res. 2002;49:45–8.
Nieto-Barrera M, Candau R, Nieto-Jimenez M, Correa A, del Portal LR. Topiramate in the treatment of severe myoclonic epilepsy in infancy. Seizure. 2000;9:590–4.
Kroll-Seger J, Portilla P, Dulac O, Chiron C. Topiramate in the treatment of highly refractory patients with Dravet syndrome. Neuropediatrics. 2006;37:325–9.
Takahashi H, Takahashi Y, Mine J, et al. Effectiveness of topiramate in eleven patients with Dravet syndrome. No To Hattatsu. 2010;42:273–6.
Striano P, Coppola A, Pezzella M, et al. An open-label trial of levetiracetam in severe myoclonic epilepsy of infancy. Neurology. 2007;69:250–4.
Liu F, Peng J, Zhu C, et al. Efficacy of the ketogenic diet in Chinese children with Dravet syndrome: a focus on neuropsychological development. Epilepsy Behav. 2019;92:98–102.
Caraballo RH. Nonpharmacologic treatments of Dravet syndrome: focus on the ketogenic diet. Epilepsia. 2011;52(Suppl. 2):79–82.
Yan N, Xin-Hua W, Lin-Mei Z, et al. Prospective study of the efficacy of a ketogenic diet in 20 patients with Dravet syndrome. Seizure. 2018;60:144–8.
Laux L, Blackford R. The ketogenic diet in Dravet syndrome. J Child Neurol. 2013;28:1041–4.
Nabbout R, Copioli C, Chipaux M, et al. Ketogenic diet also benefits Dravet syndrome patients receiving stiripentol: a prospective pilot study. Epilepsia. 2011;52:e54–7.
Kang HC, Kim YJ, Kim DW, Kim HD. Efficacy and safety of the ketogenic diet for intractable childhood epilepsy: korean multicentric experience. Epilepsia. 2005;46:272–9.
Kim JM. Ketogenic diet: old treatment, new beginning. Clin Neurophysiol Pract. 2017;2:161–2.
Ali R, Elsayed M, Kaur M, et al. Use of social media to assess the effectiveness of vagal nerve stimulation in Dravet syndrome: a caregiver’s perspective. J Neurol Sci. 2017;375:146–9.
Orosz I, McCormick D, Zamponi N, et al. Vagus nerve stimulation for drug-resistant epilepsy: a European long-term study up to 24 months in 347 children. Epilepsia. 2014;55:1576–84.
Bremer A, Lossius MI, Nakken KO. Dravet syndrome; considerable delay in making the diagnosis. Acta Neurol Scand. 2012;125:359–62.
Zamponi N, Passamonti C, Cappanera S, Petrelli C. Clinical course of young patients with Dravet syndrome after vagal nerve stimulation. Eur J Paediatr Neurol. 2011;15:8–14.
Griffin A, Hamling KR, Knupp K, Hong S, Lee LP, Baraban SC. Clemizole and modulators of serotonin signalling suppress seizures in Dravet syndrome. Brain. 2017;140:669–83.
Baraban SC, Dinday MT, Hortopan GA. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat Commun. 2013;4:2410.
Wong JC, Dutton SB, Collins SD, Schachter S, Escayg A. Huperzine A provides robust and sustained protection against induced seizures in Scn1a mutant mice. Front Pharmacol. 2016;7:357.
Dinday MT, Baraban SC. Large-scale phenotype-based antiepileptic drug screening in a zebrafish model of Dravet syndrome. eNeuro. 2015. https://doi.org/10.1523/eneuro.0068-15.2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflict of interest
Elaine C. Wirrell has received consulting fees from Biocodex. Rima Nabbout has received consulting fees from Biocodex, Zogenix, GW Pharma, Nutricia, Novartis, Stoke, Takeda, Biogene, UCB, Eisai, and Advicenne.
Rights and permissions
About this article
Cite this article
Wirrell, E.C., Nabbout, R. Recent Advances in the Drug Treatment of Dravet Syndrome. CNS Drugs 33, 867–881 (2019). https://doi.org/10.1007/s40263-019-00666-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-019-00666-8