Abstract
Background
Spinal muscular atrophy (SMA) is a neuromuscular disorder classified into four types based on the age of onset of the disease. Early onset is correlated with a higher mortality rate, mainly due to respiratory complications. Valproic acid (VPA) is a histone deacetylase (HDAC) inhibitor that has shown positive results on SMA both in experimental and cohort studies.
Objectives
This systematic review and meta-analysis aimed to investigate the efficacy and safety of VPA in patients with SMA.
Methods
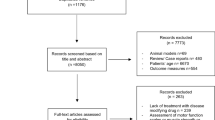
Eleven databases were systematically searched on 30 May 2017 for clinical trials that reported the efficacy and safety of VPA in SMA patients. The primary outcome was the efficacy of VPA in terms of gross motor function and expression of both full-length spinal motor neuron (SMN) gene (FL-SMN) and exon 7-lacking SMN. The secondary outcome was the safety of VPA in terms of reported adverse effects. The protocol was registered at PROSPERO (CRD42017067203).
Results
Five of the ten included studies were used in the meta-analysis (n = 126). The overall effect estimate, comparing pre- and post-VPA treatment, regardless of carnitine co-administration and design of the studies, showed significant improvement in gross motor function (standard mean difference [SMD] = 0.302, 95% confidence interval [CI] 0.048–0.556, P = 0.02) using the Hammersmith Functional Motor Scale (HFMS), Modified Hammersmith Functional Motor Scale (MHFMS), and MHFMS-Extend, with no significant heterogeneity. Similarly, in non-randomized controlled studies, the results indicated that there was a significant improvement detected (SMD = 0.335, 95% CI 0.041–0.628, P = 0.025), with no significant heterogeneity. Meanwhile, our results suggest that there was no significant improvement in treatment with co-administered carnitine (SMD = 0.28, 95% CI − 0.02 to 0.581, P = 0.067). No significant differences were found between pre- and post-VPA treatment co-administered with carnitine, in terms of the change in FL-SMN and exon 7-lacking SMN. Qualitative synthesis showed that other motor functions were not improved, while respiratory function test results were contradictory. Regarding the safety of the treatment, a double-blind, randomized, placebo-controlled trial reported no statistically significant differences for adverse events (AEs) between groups. Moreover, most of the included studies reported no serious AEs related to VPA use, although weight gain, gastrointestinal symptoms and respiratory symptoms were notable problems.
Conclusions
Our study suggests that VPA treatment results in an improvement in gross motor functions for SMA patients, but not in other assessments of motor function or, possibly, in respiratory function. Furthermore, VPA appears to be a relatively safe drug, although treatment may be associated with a wide range of AEs (including body weight increase, fatigue, fever, flu-like symptoms, irritability, and pain). Double-blind, randomized, controlled trials are required to confirm these findings.





Similar content being viewed by others
References
Tizzano EF, Zafeiriou D. Prenatal aspects in spinal muscular atrophy: from early detection to early presymptomatic intervention. Eur J Paediatr Neurol 2018;22(6):944–50.
Govoni A, et al. Time is motor neuron: therapeutic window and its correlation with pathogenetic mechanisms in spinal muscular atrophy. Mol Neurobiol. 2018;55(8):6307–18.
Verhaart IEC, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy—a literature review. Orphanet J Rare Dis. 2017;12(1):124.
Mostacciuolo ML, et al. Epidemiology of spinal muscular atrophies in a sample of the Italian population. Neuroepidemiology. 1992;11(1):34–8.
Wirth B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum Mutat. 2000;15(3):228–37.
Howell MD, Singh NN, Singh RN. Advances in therapeutic development for spinal muscular atrophy. Future Med Chem. 2014;6(9):1081–99.
Russman BS. Spinal muscular atrophy: clinical classification and disease heterogeneity. J Child Neurol. 2007;22(8):946–51.
Arnold WD, Burghes AHM. Spinal muscular atrophy: development and implementation of potential treatments. Ann Neurol. 2013;74(3):348–62.
Lefebvre S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–65.
Melki J, et al. Prenatal prediction of Werdnig-Hoffmann disease using linked polymorphic DNA probes. J Med Genet. 1992;29(3):171–4.
Brzustowicz L, et al. Genetic mapping of chronic childhood-onset spinal muscular atrophy to chromosome 5q1 1.2–13.3. Nature. 1990;344(6266):540.
Hahnen E, et al. Molecular analysis of candidate genes on chromosome 5q13 in autosomal recessive spinal muscular atrophy: evidence of homozygous deletions of the SMN gene in unaffected individuals. Hum Mol Genet. 1995;4(10):1927–33.
Finkel RS, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388(10063):3017–26.
Corey DR. Nusinersen, an antisense oligonucleotide drug for spinal muscular atrophy. Nat Neurosci. 2017;20(4):497.
Kernochan LE, et al. The role of histone acetylation in SMN gene expression. Hum Mol Genet. 2005;14(9):1171–82.
Perucca E. Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS Drugs. 2002;16(10):695–714.
Balfour JA, Bryson HM. Valproic acid. CNS Drugs. 1994;2(2):144–73.
Löscher W. Basic pharmacology of valproate. CNS Drugs. 2002;16(10):669–94.
Sumner CJ, et al. Valproic acid increases SMN levels in spinal muscular atrophy patient cells. Ann Neurol Off J Am Neurol Assoc Child Neurol Soc. 2003;54(5):647–54.
Brichta L, et al. Valproic acid increases the SMN2 protein level: a well-known drug as a potential therapy for spinal muscular atrophy. Hum Mol Genet. 2003;12(19):2481–9.
Tsai L-K, et al. Establishing a standardized therapeutic testing protocol for spinal muscular atrophy. Neurobiol Dis. 2006;24(2):286–95.
Weihl CC, Connolly AM, Pestronk A. Valproate may improve strength and function in patients with type III/IV spinal muscle atrophy. Neurology. 2006;67(3):500–1.
Tsai LK, et al. Valproic acid treatment in six patients with spinal muscular atrophy. Eur J Neurol. 2007;14(12):e8–9.
Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;21(339):b2700.
Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions, 3rd edn. Hoboken: John Wiley & Sons; 2013.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33(1):159–74.
Higgins JPT, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Sterne JAC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions, vol. 4. New York: Wiley; 2011.
Wan X, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135.
Balk EM, et al. AHRQ methods for effective health care. In: Empirical assessment of within-arm correlation imputation in trials of continuous outcomes. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012.
Brichta L, et al. In vivo activation of SMN in spinal muscular atrophy carriers and patients treated with valproate. Ann Neurol. 2006;59(6):970–5.
Darbar IA, et al. Evaluation of muscle strength and motor abilities in children with type II and III spinal muscle atrophy treated with valproic acid. BMC Neurol. 2011;11(1):36.
Kissel JT, et al. SMA valiant trial: a prospective, double-blind, placebo-controlled trial of valproic acid in ambulatory adults with spinal muscular atrophy. Muscle Nerve. 2014;49(2):187–92.
Kissel JT, et al. SMA carni-VAL trial part II: a prospective, single-armed trial of l-carnitine and valproic acid in ambulatory children with spinal muscular atrophy. PLoS One. 2011;6(7):e21296.
Piepers S, et al. Quantification of SMN protein in leucocytes from spinal muscular atrophy patients: effects of treatment with valproic acid. J Neurol Neurosurg Psychiatry. 2011;82(8):850–2.
Renusch SR, et al. Spinal Muscular Atrophy biomarker measurements from blood samples in a clinical trial of valproic acid in ambulatory adults. J Neuromuscul Dis. 2015;2(2):119–30.
Saito T, et al. A Study of valproic acid for patients with spinal muscular atrophy. Neurol Clin Neurosci. 2015;3(2):49–57.
Swoboda KJ, et al. SMA CARNI-VAL trial part I: double-blind, randomized, placebo-controlled trial of L-carnitine and valproic acid in spinal muscular atrophy. PLoS One. 2010;5(8):e12140.
Swoboda KJ, et al. Phase II open label study of valproic acid in spinal muscular atrophy. PLoS One. 2009;4(5):e5268.
Krosschell KJ, et al. Clinical trial of l-carnitine and valproic acid in spinal muscular atrophy type I. Muscle Nerve. 2018;57(2):193–9.
Krosschell KJ, et al. Reliability of the Modified Hammersmith Functional Motor Scale in young children with spinal muscular atrophy. Muscle Nerve. 2011;44(2):246–51.
Sumner CJ, et al. Valproic acid increases SMN levels in spinal muscular atrophy patient cells. Ann Neurol. 2003;54(5):647–54.
Sugai F, et al. Benefit of valproic acid in suppressing disease progression of ALS model mice. Eur J Neurosci. 2004;20(11):3179–83.
Bezkorovainy A. Carnosine, carnitine, and Vladimir Gulevich. J Chem Educ. 1974;51:652–4.
Winter BK, Fiskum G, Gallo LL. Effects of l-carnitine on serum triglyceride and cytokine levels in rat models of cachexia and septic shock. Br J Cancer. 1995;72:1173–9.
Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol (Phila). 2009;47:101–11.
Silva MF, Aires CC, Luis PB. Valproic acid metabolism and its effects on mitochondrial fatty acid oxidation. A review. J Inherit Metabol Dis. 2008;31:205–16.
Kang S-W, Bach JR. Maximum insufflation capacity: vital capacity and cough flows in neuromuscular disease. Am J Phys Med Rehabil. 2000;79(3):222–7.
Crawford TO, et al. Abnormal fatty acid metabolism in childhood spinal muscular atrophy. Ann Neurol. 1999;45(3):337–43.
Tein I, et al. Fatty acid oxidation abnormalities in childhood-onset spinal muscular atrophy: primary or secondary defect(s)? Pediatr Neurol. 1995;12(1):21–30.
Coulter D. Carnitine deficiency: a possible mechanism for valproate hepatotoxicity. Lancet. 1984;323(8378):689.
Garbes L, et al. VPA response in SMA is suppressed by the fatty acid translocase CD36. Hum Mol Genet. 2013;22(2):398–407.
Acknowledgements
This study was conducted (in part) at the Joint Usage/Research Center on Tropical Disease, Institute of Tropical Medicine, Nagasaki University, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for preparation of this article.
Conflict of interests
All authors, AE, THH, MFD, MAMK, MFE, SKH, HA, KH, and NTH, declare no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Elshafay, A., Hieu, T.H., Doheim, M.F. et al. Efficacy and Safety of Valproic Acid for Spinal Muscular Atrophy: A Systematic Review and Meta-Analysis. CNS Drugs 33, 239–250 (2019). https://doi.org/10.1007/s40263-019-00606-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-019-00606-6