Abstract
Background
Concerns about the generalizability of pharmacotherapy efficacy trials to “real-world” patients have been raised for more than 40 years. Almost all of this literature has focused on treatment studies of major depressive disorder (MDD).
Objective
The aim of the study was to review the psychiatric inclusion and exclusion criteria used in placebo-controlled trials that assessed the efficacy of medications for bipolar depression (bipolar disorder efficacy trials [BDETs]) and compare the criteria used in BDETs with those used in efficacy trials of antidepressants to treat MDD (antidepressant efficacy trials [AETs]).
Methods
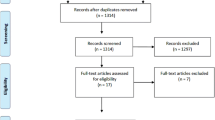
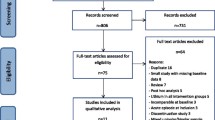
We searched the MEDLINE, Embase, and PsycINFO databases for articles published from January 1995 through December 2014. We identified 170 placebo-controlled AETs and 22 BDETs published during these 20 years. Two of the authors independently reviewed each article and completed a pre-specified information extraction form listing the psychiatric inclusion and exclusion criteria used in the study.
Results
Six inclusion/exclusion criteria were used in at least half of the BDETs: minimum severity on a depression symptom severity scale, significant suicidal ideation, diagnosis of alcohol or drug use disorder, presence of a comorbid nondepressive, nonsubstance use Axis I disorder, current episode of depression being too long, and absence of current manic symptoms. BDETs were significantly less likely than AETs to exclude patients with a history of psychotic features/disorders, borderline personality disorder, and post-traumatic stress disorder and more likely to exclude individuals who scored too low on the first item of the Hamilton Depression Rating Scale. Nearly two-thirds of the BDETs placed an upper limit on the duration of the current depressive episode, three times higher than the rate in the AETs. There was no difference on other variables between the AETs and BDETs.
Conclusions
Similar to treatment studies of nonbipolar MDD, the treatment studies of bipolar depression frequently excluded patients with comorbid psychiatric and substance use disorders and insufficient severity of depressive symptoms as rated on standardized scales. These findings indicate that concerns about the generalizability of data from trials of recently approved medications for the treatment of bipolar depression are as relevant as the concerns that have been raised about studies of antidepressants for nonbipolar depression.
Similar content being viewed by others
References
Brauzer B, Goldstein BJ. Symptomic volunteers: another patient dimension for clinical trials. J Clin Pharmacol. 1973;13:89–98.
Breckenridge JS, Zeiss AM, Breckenridge JN, Gallagher D, Thompson LW. Solicitations of elderly depressives for treatment outcome research: a comparison of referral sources. J Consult Clin Psychol. 1985;53:552–4.
Miller CA, Hooper CL, Bakish D. A comparison of patients with major depressive disorder recruited through newspaper advertising versus consultation referrals for clinical drug trials. Psychopharmacol Bull. 1997;33:69–73.
Rapaport MH, Zisook S, Frevert T, Seymour S, Kelsoe JR, Judd LJ. A comparison of descriptive variables for clinical patients and symptomatic volunteers with depressive disorders. J Clin Psychopharmacol. 1996;16:242–6.
Covi L, Lipman R, McNair D, Czerlinsky T. Symptomatic volunteers in multicenter drug trails. Neuropsychopharmacol. 1979;3:521–33.
Partonen T, Sihvo S, Lonnqvist JK. Patients excluded from antidepressant efficacy trial. J Clin Psychiatry. 1996;57:572–5.
Keitner G, Posternak M, Ryan C. How many subjects with major depressive disorder meet eligibility requirements of an antidepressant efficacy trial? J Clin Psychiatry. 2003;64:1091–3.
Zimmerman M, Mattia JI, Posternak MA. Are subjects in pharmacological treatment trials of depression representative of patients in routine clinical practice. Am J Psychiatry. 2002;159:469–73.
van der Lem R, van der Wee NJ, van Veen T, Zitman FG. The generalizability of antidepressant efficacy trials to routine psychiatric out-patient practice. Psychol Med. 2011;41:1353–63.
Wisniewski SR, Rush AJ, Nierenberg AA, Gaynes BN, Warden D, Luther JF, et al. Can phase III trial results of antidepressant medications be generalized to clinical practice? A STAR*D report. Am J Psychiatry. 2009;166:599–607.
Zetin M, Hoepner CT. Relevance of exclusion criteria in antidepressant clinical trials: a replication study. J Clin Psychopharmacol. 2007;27:295–301.
Zimmerman M, Chelminski I, Posternak M. Exclusion criteria used in antidepressant efficacy trials: consistency across studies and representativeness of samples included. J Nerv Ment Dis. 2004;192:87–94.
Zimmerman M, Clark HL, Multach MD, Walsh E, Rosenstein LK, Gazarian D. Have treatment studies of depression become even less generalizable?: a review of the inclusion and exclusion criteria in placebo controlled antidepressant efficacy trials published during the past 20 years. Mayo Clin Proceed. 2015;90:1180–6.
Amsterdam J, Shults J. Comparison of fluoxetine, olanzapine, and combined fluoxetine plus olanzapine initial therapy of bipolar type I and type II major depression–lack of manic induction. J Affect Disord. 2005;87:121–30.
Calabrese J, Bowden C, Sachs G, Ascher J, Monaghan E, Rudd G. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999;60:79–88.
Calabrese J, Keck P, Macfadden W, Minkwitz M, Ketter T, Weisler R, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 2005;162:1351–60.
Calabrese J, Huffman R, White R, Edwards S, Thompson T, Ascher J, et al. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disord. 2008;10:323–33.
Davis L, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord. 2005;85:259–66.
Ghaemi S, Gilmer W, Goldberg J, Zablotsky B, Kemp D, Kelley M, et al. Divalproex in the treatment of acute bipolar depression: a preliminary double-blind, randomized, placebo-controlled pilot study. J Clin Psychiatry. 2007;68:1840–4.
Lombardo I, Sachs G, Kolluri S, Kremer C, Yang R. Two 6-week, randomized, double-blind, placebo-controlled studies of ziprasidone in outpatients with bipolar I depression: did baseline characteristics impact trial outcome? J Clin Psychopharmacol. 2012;32:470–8.
Loebel A, Cucchiaro J, Silva R, Kroger H, Hsu J, Sarma K, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171:160–8.
McElroy S, Weisler R, Chang W, Olausson B, Paulsson B, Brecher M, et al. A double-blind, placebo-controlled study of quetiapine and paroxetine as monotherapy in adults with bipolar depression (EMBOLDEN II). J Clin Psychiatry. 2010;71:163–74.
Muzina D, Gao K, Kemp D, Khalife S, Ganocy S, Chan P, et al. Acute efficacy of divalproex sodium versus placebo in mood stabilizer-naive bipolar I or II depression: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2011;72:813–9.
Suppes T, Datto C, Minkwitz M, Nordenhem A, Walker C, Darko D. Effectiveness of the extended release formulation of quetiapine as monotherapy for the treatment of acute bipolar depression. J Affect Disord. 2014;168:485–93.
Thase M, Macfadden W, Weisler R, Chang W, Paulsson B, Khan A, et al. Efficacy of quetiapine monotherapy in bipolar I and II depression: a double-blind, placebo-controlled study (the BOLDER II study). J Clin Psychopharmacol. 2006;26:600–9.
Thase M, Jonas A, Khan A, Bowden C, Wu X, McQuade R, et al. Aripiprazole monotherapy in nonpsychotic bipolar I depression: results of 2 randomized, placebo-controlled studies. J Clin Psychopharmacol. 2008;28:13–20.
Tohen M, Vieta E, Calabrese J, Ketter T, Sachs G, Bowden C, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry. 2003;60:1079–88.
Tohen M, McDonnell D, Case M, Kanba S, Ha K, Fang Y, et al. Randomised, double-blind, placebo-controlled study of olanzapine in patients with bipolar I depression. Br J Psychiatry. 2012;201:376–82.
Wang M, Tong J, Huang D, Zhu G, Liang G, Du H. Efficacy of olanzapine monotherapy for treatment of bipolar I depression: a randomized, double-blind, placebo controlled study. Psychopharmacology. 2014;231:2811–8.
Young A, McElroy S, Bauer M, Philips N, Chang W, Olausson B, et al. A double-blind, placebo-controlled study of quetiapine and lithium monotherapy in adults in the acute phase of bipolar depression (EMBOLDEN I). J Clin Psychiatry. 2010;71:150–62.
Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9.
Goes FS, Sadler B, Toolan J, Zamoiski RD, Mondimore FM, MacKinnon DF, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9:901–6.
Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252–60.
Ghaemi SN. The failure to know what isn’t known: negative publication bias with lamotrigine and a glimpse inside peer review. Evid Based Ment Health. 2009;12:65–8.
Koesters M, Guaiana G, Cipriani A, Becker T, Barbui C. Agomelatine efficacy and acceptability revisited: systematic review and meta-analysis of published and unpublished randomised trials. Br J Psychiatry. 2013;203:179–87.
Taylor D, Sparshatt A, Varma S, Olofinjana O. Antidepressant efficacy of agomelatine: meta-analysis of published and unpublished studies. BMJ. 2014;348:g1888.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding source
No sources of funding were used to conduct this study or prepare this manuscript.
Conflicts of interest
M Zimmerman has participated on advisory boards for F. Hoffmann-La Roche, Genentech, and Lundbeck; received research support from Azevan and Eli Lilly; and prepared educational material for Otsuka. Ms. Holst, Ms. Clark, Mr. Multach, Ms. Walsh, Ms. Rosenstein, and Mr. Gazarian do not have any conflicts.
Rights and permissions
About this article
Cite this article
Zimmerman, M., Holst, C.G., Clark, H.L. et al. The Psychiatric Inclusion and Exclusion Criteria in Placebo-Controlled Monotherapy Trials of Bipolar Depression: An Analysis of Studies of the Past 20 Years. CNS Drugs 30, 1209–1218 (2016). https://doi.org/10.1007/s40263-016-0381-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-016-0381-0