Abstract
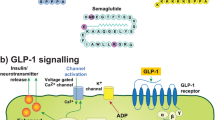
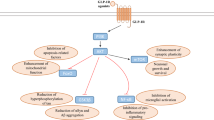
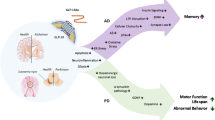
The link between diabetes mellitus and Alzheimer’s disease (AD) has been known for the last few decades. Since insulin and insulin receptors are known to be present in the brain, the downstream signalling as well as the effect of hyperinsulinemia have been extensively studied in both AD and Parkinson’s disease. Glucagon-like peptide-1 (GLP-1) is a hormone belonging to the incretin family, and its receptors (GLP-1Rs) can be found in pancreatic cells and in vascular endothelium. Interestingly, GLP-1Rs are found in the neuronal cell body and dendrites in the central nervous system (CNS), in particular in the hypothalamus, hippocampus, cerebral cortex and olfactory bulb. Several studies have shown the importance of both insulin and GLP-1 signalling on cognitive function, and many preclinical studies have been performed to evaluate the potential protective role of GLP-1 on the brain. Here we review the underlying mechanism of insulin and GLP-1 signalling in the CNS, as well as the preclinical data for the use of GLP-1 analogues such as liraglutide, exenatide and lixisenatide in neurodegenerative diseases.







Similar content being viewed by others
References
Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75 (e2).
Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137–52.
Alzheimer’s Association 2012. Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8(2):131–68.
de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2(6):1101–13.
Hoyer S, Nitsch R, Oesterreich K. Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: a cross-sectional comparison against advanced late-onset and incipient early-onset cases. J Neural Transm Park Dis Dement Sect. 1991;3(1):1–14.
Hoyer S, Oesterreich K, Wagner O. Glucose metabolism as the site of the primary abnormality in early-onset dementia of Alzheimer type? J Neurol. 1988;235(3):143–8.
Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm. 1998;105(4–5):423–38.
Schwab RS. Progression and prognosis in Parkinson’s disease. J Nerv Ment Dis. 1960;130:556–66.
Boyd AE 3rd, Lebovitz HE, Feldman JM. Endocrine function and glucose metabolism in patients with Parkinson’s disease and their alternation by l-dopa. J Clin Endocrinol Metab. 1971;33(5):829–37.
Van Woert MW, Mueller PS. Glucose, insulin, and free fatty acid metabolism in Parkinson’s disease treated with levodopa. Clin Pharmacol Ther. 1971;12(2):360–7.
Havrankova J, Schmechel D, Roth J, Brownstein M. Identification of insulin in rat brain. Proc Natl Acad Sci USA. 1978;75(11):5737–41.
Hoyer S, Henneberg N, Knapp S, Lannert H, Martin E. Brain glucose metabolism is controlled by amplification and desensitization of the neuronal insulin receptor. Ann N Y Acad Sci. 1996;777:374–9.
Barone E, Butterfield AD. Insulin resistance in Alzheimer disease: Is heme oxygenase-1 an Achille’s heel? Neurobiol Dis. Epub. 2015. doi:10.1016/j.nbd.2015.02.013.
Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology. 1999;53(9):1937–42.
De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63(7):2262–72.
Ohara T, Doi Y, Ninomiya T, Hirakawa Y, Hata J, Iwaki T, et al. Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology. 2011;77(12):1126–34.
Yu LY, Pei Y. Insulin neuroprotection and the mechanisms. Chin Med J (Engl). 2015;128(7):976–81.
Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58(5):708–19.
De Felice FG, Lourenco MV, Ferreira ST. How does brain insulin resistance develop in Alzheimer’s disease? Alzheimers Dement. 2014;10(1 Suppl):S26–32.
Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010;31(2):224–43.
Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim Biophys Acta. 2009;1792(5):482–96.
Hoyer S, Lee SK, Loffler T, Schliebs R. Inhibition of the neuronal insulin receptor. An in vivo model for sporadic Alzheimer disease? Ann N Y Acad Sci. 2000;920:256–8.
Petrov D, Pedros I, Artiach G, Sureda FX, Barroso E, Pallas M, et al. High-fat diet-induced deregulation of hippocampal insulin signaling and mitochondrial homeostasis deficiences contribute to Alzheimer disease pathology in rodents. Biochim Biophys Acta. 2015;1852(9):1687–99.
Reagan LP. Neuronal insulin signal transduction mechanisms in diabetes phenotypes. Neurobiol Aging. 2005;26(Suppl 1):56–9.
McNay EC, Recknagel AK. Brain insulin signaling: a key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol Learn Mem. 2011;96(3):432–42.
McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010;93(4):546–53.
Crescenzo R, Bianco F, Mazzoli A, Giacco A, Liverini G, Iossa S. Mitochondrial efficiency and insulin resistance. Front Physiol. 2014;5:512.
Carvalho C, Santos MS, Oliveira CR, Moreira PI. Alzheimer’s disease and type 2 diabetes-related alterations in brain mitochondria, autophagy and synaptic markers. Biochim Biophys Acta. 2015;1852(8):1665–75.
Carvalho C, Cardoso S, Correia SC, Santos RX, Santos MS, Baldeiras I, et al. Metabolic alterations induced by sucrose intake and Alzheimer’s disease promote similar brain mitochondrial abnormalities. Diabetes. 2012;61(5):1234–42.
Dineley KT, Jahrling JB, Denner L. Insulin resistance in Alzheimer’s disease. Neurobiol Dis. 2014;72 (Pt A):92–103.
Cabou C, Burcelin R. GLP-1, the gut-brain, and brain-periphery axes. Rev Diabet Stud. 2011;8(3):418–31.
Anagnostis P, Athyros VG, Adamidou F, Panagiotou A, Kita M, Karagiannis A, et al. Glucagon-like peptide-1-based therapies and cardiovascular disease: looking beyond glycaemic control. Diabetes Obes Metab. 2011;13(4):302–12.
Wideman RD, Kieffer TJ. Mining incretin hormone pathways for novel therapies. Trends Endocrinol Metab. 2009;20(6):280–6.
Holscher C. The incretin hormones glucagonlike peptide 1 and glucose-dependent insulinotropic polypeptide are neuroprotective in mouse models of Alzheimer’s disease. Alzheimers Dement. 2014;10(1 Suppl):S47–54.
Gejl M, Rungby J, Brock B, Gjedde A. At the centennial of Michaelis and Menten, competing Michaelis-Menten steps explain effect of GLP-1 on blood-brain transfer and metabolism of glucose. Basic Clin Pharmacol. 2014;115(2):162–71.
Gejl M, Egefjord L, Lerche S, Vang K, Bibby BM, Holst JJ, et al. Glucagon-like peptide-1 decreases intracerebral glucose content by activating hexokinase and changing glucose clearance during hyperglycemia. J Cereb Blood Flow Metab. 2012;32(12):2146–52.
Pabreja K, Mohd MA, Koole C, Wootten D, Furness SG. Molecular mechanisms underlying physiological and receptor pleiotropic effects mediated by GLP-1R activation. Br J Pharmacol. 2014;171(5):1114–28.
Sonoda N, Imamura T, Yoshizaki T, Babendure JL, Lu JC, Olefsky JM. Beta-Arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic beta cells. Proc Natl Acad Sci USA. 2008;105(18):6614–9.
Holscher C, Li L. New roles for insulin-like hormones in neuronal signalling and protection: new hopes for novel treatments of Alzheimer’s disease? Neurobiol Aging. 2010;31(9):1495–502.
Lund A, Knop FK, Vilsboll T. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: differences and similarities. Eur J Intern Med. 2014;25(5):407–14.
Struble RG, Ala T, Patrylo PR, Brewer GJ, Yan XX. Is brain amyloid production a cause or a result of dementia of the Alzheimer’s type? J Alzheimers Dis. 2010;22(2):393–9.
Varnum MM, Ikezu T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain. Arch Immunol Ther Exp (Warsz). 2012;60(4):251–66.
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–80.
Wilcox KC, Lacor PN, Pitt J, Klein WL. Abeta oligomer-induced synapse degeneration in Alzheimer’s disease. Cell Mol Neurobiol. 2011;31(6):939–48.
Freude S, Plum L, Schnitker J, Leeser U, Udelhoven M, Krone W, et al. Peripheral hyperinsulinemia promotes tau phosphorylation in vivo. Diabetes. 2005;54(12):3343–8.
Moroo I, Yamada T, Makino H, Tooyama I, McGeer PL, McGeer EG, et al. Loss of insulin receptor immunoreactivity from the substantia nigra pars compacta neurons in Parkinson’s disease. Acta Neuropathol. 1994;87(4):343–8.
Takahashi M, Yamada T, Tooyama I, Moroo I, Kimura H, Yamamoto T, et al. Insulin receptor mRNA in the substantia nigra in Parkinson’s disease. Neurosci Lett. 1996;204(3):201–4.
Morris JK, Bomhoff GL, Gorres BK, Davis VA, Kim J, Lee PP, et al. Insulin resistance impairs nigrostriatal dopamine function. Exp Neurol. 2011;231(1):171–80.
Morales I, Guzman-Martinez L, Cerda-Troncoso C, Farias GA, Maccioni RB. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci. 2014;8:112.
Hoozemans JJ, Veerhuis R, Rozemuller JM, Eikelenboom P. Neuroinflammation and regeneration in the early stages of Alzheimer’s disease pathology. Int J Dev Neurosci. 2006;24(2–3):157–65.
Heneka MT, Sastre M, Dumitrescu-Ozimek L, Dewachter I, Walter J, Klockgether T, et al. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J Neuroinflammation. 2005;2:22.
Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int J Dev Neurosci. 2006;24(2–3):167–76.
Misiak B, Leszek J, Kiejna A. Metabolic syndrome, mild cognitive impairment and Alzheimer’s disease–the emerging role of systemic low-grade inflammation and adiposity. Brain Res Bull. 2012;89(3–4):144–9.
Lee YH, Tharp WG, Maple RL, Nair S, Permana PA, Pratley RE. Amyloid precursor protein expression is upregulated in adipocytes in obesity. Obesity. 2008;16(7):1493–500.
Ho GJ, Drego R, Hakimian E, Masliah E. Mechanisms of cell signaling and inflammation in Alzheimer’s disease. Curr Drug Targets Inflamm Allergy. 2005;4(2):247–56.
Stojkovska I, Wagner BM, Morrison BE. Parkinson’s disease and enhanced inflammatory response. Exp Biol Med (Maywood). 2015;240(11):1387–95.
Iwai T, Ito S, Tanimitsu K, Udagawa S, Oka J. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci Res. 2006;55(4):352–60.
Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9(11):1118–27.
Femminella GD, Edison P. Evaluation of neuroprotective effect of glucagon-like peptide 1 analogs using neuroimaging. Alzheimers Dement. 2014;10(1 Suppl):S55–61.
Okello A, Koivunen J, Edison P, Archer HA, Turkheimer FE, Nagren K, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73(10):754–60.
Edison P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE, et al. Microglia, amyloid, and cognition in Alzheimer’s disease: An [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis. 2008;32(3):412–9.
Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95(11):6448–53.
Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–9.
Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, et al. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24(45):10191–200.
De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, et al. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci USA. 2009;106(6):1971–6.
Pandini G, Pace V, Copani A, Squatrito S, Milardi D, Vigneri R. Insulin has multiple antiamyloidogenic effects on human neuronal cells. Endocrinology. 2013;154(1):375–87.
Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, et al. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002;300(3):958–66.
Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, Egan JM, et al. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res. 2003;72(5):603–12.
Perry T, Holloway HW, Weerasuriya A, Mouton PR, Duffy K, Mattison JA, et al. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp Neurol. 2007;203(2):293–301.
During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9(9):1173–9.
Abbas T, Faivre E, Holscher C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: interaction between type 2 diabetes and Alzheimer’s disease. Behav Brain Res. 2009;205(1):265–71.
D’Amico M, Di Filippo C, Marfella R, Abbatecola AM, Ferraraccio F, Rossi F, et al. Long-term inhibition of dipeptidyl peptidase-4 in Alzheimer’s prone mice. Exp Gerontol. 2010;45(3):202–7.
Kosaraju J, Gali CC, Khatwal RB, Dubala A, Chinni S, Holsinger RM, et al. Saxagliptin: a dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer’s disease. Neuropharmacology. 2013;72:291–300.
Kosaraju J, Murthy V, Khatwal RB, Dubala A, Chinni S, Muthureddy Nataraj SK, et al. Vildagliptin: an anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer’s disease. J Pharm Pharmacol. 2013;65(12):1773–84.
Kornelius E, Lin CL, Chang HH, Li HH, Huang WN, Yang YS, et al. DPP-4 inhibitor linagliptin attenuates Abeta-induced cytotoxicity through activation of AMPK in neuronal cells. CNS Neurosci Ther. 2015;21(7):549–57.
Jaturapatporn D, Isaac MG, McCleery J, Tabet N. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane Database Syst Rev. 2012;2:CD006378.
Shie FS, Nivison M, Hsu PC, Montine TJ. Modulation of microglial innate immunity in Alzheimer’s disease by activation of peroxisome proliferator-activated receptor gamma. Curr Med Chem. 2009;16(6):643–51.
Heneka MT, Landreth GE. PPARs in the brain. Biochim Biophys Acta. 2007;1771(8):1031–45.
Risner ME, Saunders AM, Altman JFB, Ormandy GC, Craft S, Foley IM, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 2006;6(4):246–54.
Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone—a preliminary study. Am J Geriat Psychiatry. 2005;13(11):950–8.
Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging. 2011;32(9):1626–33.
Shiraishi D, Fujiwara Y, Komohara Y, Mizuta H, Takeya M. Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem Biophys Res Commun. 2012;425(2):304–8.
Foltynie T, Aviles-Olmos I. Exenatide as a potential treatment for patients with Parkinson’s disease: first steps into the clinic. Alzheimers Dement. 2014;10(1 Suppl):S38–46.
Rampersaud N, Harkavyi A, Giordano G, Lever R, Whitton J, Whitton PS. Exendin-4 reverses biochemical and behavioral deficits in a pre-motor rodent model of Parkinson’s disease with combined noradrenergic and serotonergic lesions. Neuropeptides. 2012;46(5):183–93.
Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res. 2008;86(2):326–38.
Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci USA. 2009;106(4):1285–90.
Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Kahan J, Ell P, et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis. 2014;4(3):337–44.
Liu W, Jalewa J, Sharma M, Li G, Li L, Holscher C. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience. 2015;303:42–50.
Holscher C. First clinical data of the neuroprotective effects of nasal insulin application in patients with Alzheimer’s disease. Alzheimers Dement. 2014;10(1 Suppl):S33–7.
Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69(1):29–38.
Yang Y, Ma D, Wang Y, Jiang T, Hu S, Zhang M, et al. Intranasal insulin ameliorates tau hyperphosphorylation in a rat model of type 2 diabetes. J Alzheimers Dis. 2013;33(2):329–38.
Yang Y, Zhang J, Ma D, Zhang M, Hu S, Shao S, et al. Subcutaneous administration of liraglutide ameliorates Alzheimer-associated tau hyperphosphorylation in rats with type 2 diabetes. J Alzheimers Dis. 2013;37(3):637–48.
Gengler S, McClean PL, McCurtin R, Gault VA, Holscher C. Val(8)GLP-1 rescues synaptic plasticity and reduces dense core plaques in APP/PS1 mice. Neurobiol Aging. 2012;33(2):265–76.
Bomba M, Ciavardelli D, Silvestri E, Canzoniero LM, Lattanzio R, Chiappini P, et al. Exenatide promotes cognitive enhancement and positive brain metabolic changes in PS1-KI mice but has no effects in 3xTg-AD animals. Cell Death Dis. 2013;4:e612.
Hunter K, Holscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33.
McClean PL, Parthsarathy V, Faivre E, Holscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci. 2011;31(17):6587–94.
McClean PL, Holscher C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology. 2014;76(Pt A):57–67.
McClean PL, Holscher C. Lixisenatide, a drug developed to treat type 2 diabetes, shows neuroprotective effects in a mouse model of Alzheimer’s disease. Neuropharmacology. 2014;86:241–58.
Li L, Zhang ZF, Holscher C, Gao C, Jiang YH, Liu YZ. (Val(8)) glucagon-like peptide-1 prevents tau hyperphosphorylation, impairment of spatial learning and ultra-structural cellular damage induced by streptozotocin in rat brains. Eur J Pharmacol. 2012;674(2–3):280–6.
Hansen HH, Fabricius K, Barkholt P, Niehoff ML, Morley JE, Jelsing J, et al. The GLP-1 receptor agonist liraglutide improves memory function and increases hippocampal CA1 neuronal numbers in a senescence-accelerated mouse model of Alzheimer’s disease. J Alzheimers Dis. 2015;46(4):877–88.
Kelly P, McClean PL, Ackermann M, Konerding MA, Holscher C, Mitchell CA. Restoration of cerebral and systemic microvascular architecture in APP/PS1 transgenic mice following treatment with Liraglutide. Microcirculation. 2015;22(2):133–45.
Ma DL, Chen FQ, Xu WJ, Yue WZ, Yuan G, Yang Y. Early intervention with glucagon-like peptide 1 analog liraglutide prevents tau hyperphosphorylation in diabetic db/db mice. J Neurochem. 2015;135(2):301–8.
Barkholt P, Fabricius K, Jelsing J, Terwel D, Hansen HH, Pyke C, et al. The GLP-1 analog liraglutide improves motor function, survival rate and reduces neurofibrillary tangle load in a transgenic hTauP301L tauopathy mouse model of Alzheimer’s disease [poster no. 691.02]. Neuroscience 2014. 2014 (Washington, DC). http://www.abstractsonline.com/plan/start.aspx?mkey=%7B8334BE29-8911-4991-8C31-32B32DD5E6C8%7D. Accessed 10 Dec 2015.
Imperial College London. Evaluating Liraglutide in Alzheimer’s Disease (ELAD) [ClinicalTrials.gov identifier NCT01843075]. 2013. US National Institutes of Health, ClinicalTrials.gov. http://clinicaltrials.gov. Accessed 27 Nov 2015.
Talbot K, Wang HY. The nature, significance, and glucagon-like peptide-1 analog treatment of brain insulin resistance in Alzheimer’s disease. Alzheimers Dement. 2014;10(1 Suppl):S12–25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Calsolaro has nothing to disclose. Dr. Edison received funding from Novo Nordisk Pharmaceuticals and from GE Healthcare. Dr. Edison was funded by the Medical Research Council and is currently funded by the Higher Education Funding Council for England (HEFCE). He has also received grants from Alzheimer’s Research UK, Alzheimer’s Drug Discovery Foundation, the UK Alzheimer’s Society, Novo Nordisk and GE Healthcare. Dr Calsolaro received funding from Alzheimer Research, UK.
Rights and permissions
About this article
Cite this article
Calsolaro, V., Edison, P. Novel GLP-1 (Glucagon-Like Peptide-1) Analogues and Insulin in the Treatment for Alzheimer’s Disease and Other Neurodegenerative Diseases. CNS Drugs 29, 1023–1039 (2015). https://doi.org/10.1007/s40263-015-0301-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-015-0301-8