Abstract
Background
Attention is the capacity to flexibly orient behaviors and thoughts towards a goal by selecting and integrating relevant contextual information. The dorsal cingulate (dCC) and prefrontal (PFC) cortices play critical roles in attention. Evidence indicates that catechol-O-methyltransferase (COMT) modulates dopaminergic tone in the PFC and dCC.
Objective
In this study, we explored the effect of tolcapone, a CNS penetrant COMT inhibitor that increases cortical dopamine levels, on brain activity during a Variable Attentional Control (VAC) task.
Study Design
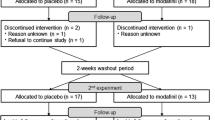
We performed a double-blinded, placebo-controlled, counter-balanced trial with tolcapone (Tasmar, tablets, 100 mg three times a day for 1 day and then 200 mg three times a day for 6 days; ClinicalTrials.gov identifier: NCT00044083).
Setting
The study was conducted in the Clinical Center of the National Institute of Mental Health from 2005 to 2009.
Patients
Twenty healthy volunteers (11 males; mean age = 32.7 years) with good imaging and performance data on both arms of the study were investigated.
Intervention
Participants underwent 3T blood-oxygen-level-dependent (BOLD) functional magnetic resonance imaging (fMRI) while performing the event-related VAC task, which varies attention over three levels of load: LOW, INT (intermediate), and HIGH.
Main Outcome Measure
Changes in behavioral data and individual contrast images were analyzed using ANOVA with drug and task load as co-factors.
Results
There was a significant main effect of increasing task load, with resulting decreased accuracy and increased reaction time. While there was no significant effect of tolcapone on these behavioral measures, the neuroimaging data showed a significant effect on load-related changes in dCC, with significantly lower dCC activation on tolcapone compared with placebo. Further, neural activity in dCC correlated positively with COMT enzyme activity (i.e., lower COMT activity and presumably more dopamine was associated with lower activation in dCC, i.e., more efficient information processing).
Conclusion
Our results show that pharmacological reduction of COMT activity modulates the engagement of attentional mechanisms, selectively enhancing the efficiency of dCC processing in healthy volunteers, reflected as decreased activity for the same level of performance.




Similar content being viewed by others
References
MacDonald AWI, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–8.
Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, Martinez A, et al. Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. NeuroImage. 2003;20(4):2135–41.
Blasi G, Goldberg TE, Elvevåg B, Rasetti R, Bertolino A, Cohen J, et al. Differentiating allocation of resources and conflict detection within attentional control processing. Eur J Neurosci. 2007;25(2):594–602.
Weissman DH, Giesbrecht B, Song AW, Mangun GR, Woldorff MG. Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. Neuroimage. 2003;19(4):1361–8.
Kerns JG, Cohen JD, MacDonald AW 3, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004; 303(5660):1023–6.
Cohen RA, Kaplan RF, Moser DJ, Jenkins MA, Wilkinson H. Impairments of attention after cingulotomy. Neurology. 1999;53(4):819–24.
Danckert J, Maruff P, Ymer C, Kinsella G, Yucel M, de Graaff S, et al. Goal-directed selective attention and response competition monitoring: Evidence from unilateral parietal and anterior cingulate lesion. Neuropsychology. 2000;14(1):16–28.
Bartus RT, Levere TE. Frontal decortication in rhesus monkeys: a test of the interference hypothesis. Brain Res. 1977;119(1):233–48.
Wilkins AJ, Shallice T, McCarthy R. Frontal lesions and sustained attention. Neuropsychologia. 1987;25(2):359–65.
Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, et al. Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain. 1992;115:1727–51.
Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995;33(1):1–24.
Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20(11):4320–4.
Deuel RK, Regan DJ. Parietal hemineglect and motor deficits in the monkey. Neuropsychologia. 1985;23(3):305–14.
King VR, Corwin JV. Comparisons of hemi-inattention produced by unilateral lesions of the posterior parietal cortex or medial agranular prefrontal cortex in rats: Neglect, extinction, and the role of stimulus distance. Behav Brain Res. 1993;54(2):117–31.
Danckert J, Stöttinger E, Quehl N, Anderson B. Right hemisphere brain damage impairs strategy updating. Cereb Cortex. 2012;22(12):2745–60.
Yücel M, Volker C, Collie A, Maruff P, Danckert J, Velakoulis D, et al. Impairments of response conflict monitoring and resolution in schizophrenia. Psychol Med. 2002;32(7):1251–60.
Weiss EM, Golaszewski S, Mottaghy FM, Hofer A, Hausmann A, Kemmler G, et al. Brain activation patterns during a selective attention test-a functional MRI study in healthy volunteers and patients with schizophrenia. Psychiatry Res. 2003;123(1):1–15.
Heckers S, Weiss AP, Deckersbach T, Goff DC, Morecraft RJ, Bush G. Anterior cingulate cortex activation during cognitive interference in schizophrenia. Am J Psychiatry. 2004;161(4):707–15.
Kerns JG, Cohen JD, MacDonald AW 3, Johnson MK, Stenger VA, Aizenstein H, et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005; 162(10): 1833–9.
Weiss EM, Siedentopf C, Golaszewski S, Mottaghy FM, Hofer A, Kremser C, et al. Brain activation patterns during a selective attention test—a functional MRI study in healthy volunteers and unmedicated patients during an acute episode of schizophrenia. Psychiatry Res. 2007;154(1):31–40.
Blasi G, Taurisano P, Papazacharias A, Caforio G, Romano R, Lobianco L, Fazio L, Di Giorgio A, Latorre V, Sambataro F, Popolizio T, Nardini M, Mattay VS, Weinberger DR, Bertolino A. Nonlinear response of the anterior cingulate and prefrontal cortex in schizophrenia as a function of variable attentional control. Cereb Cortex. 2010;20(4):837–45.
Delawalla Z, Csernansky JG, Barch DM. Prefrontal cortex function in nonpsychotic siblings of individuals with schizophrenia. Biol Psychiatry. 2008;63(5):490–7.
Cools R, Rogers R, Barker RA, Robbins TW. Top-down attentional control in Parkinson’s disease: salient considerations. J Cognitive Neurosci. 2010;22(5):848–59.
Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41.
Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67(1):53–83.
Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74(1):1–58.
Goldman-Rakic PS, Muly EC 3, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31(2–3):295–301.
Devilbiss DM, Berridge CW. Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol Psychiatry. 2008;64(7):626–35.
Daniel DG, Weinberger DR, Jones DW, Zigun JR, Coppola R, Handel S, Bigelow LB, Goldberg TE, Berman KF, Kleinman JE. The effect of amphetamine on regional cerebral blood flow during cognitive activation in schizophrenia. J Neurosci. 1991;11(7):1907–17.
Mattay VS, Berman KF, Ostrem JL, Esposito G, Van Horn JD, Bigelow LB, Weinberger DR. Dextroamphetamine enhances “neural network-specific” physiological signals: a positron-emission tomography rCBF study. J Neurosci. 1996;16(15):4816–22.
Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000; 20(6): RC65.
Tomasi D, Volkow ND, Wang GJ, Wang R, Telang F, Caparelli EC, Wong C, Jayne M, Fowler JS. Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage. 2011;54(4):3101–10.
Karoum F, Chrapusta SJ, Egan MF. 3-methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: Reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem. 1994;63(3):972–9.
Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708.
Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-O-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24(23):5331–5.
Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28(35):8709–23.
Liljequist R, Haapalinna A, Ahlander M, Li YH, Mannisto PT. Catechol O-methyltransferase inhibitor tolcapone has minor influence on performance in experimental memory models in rats. Behav Brain Res. 1997; 82(2): 202–202.
Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, et al. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology. 2007;32(5):1011–20.
Blasi G, Mattay VS, Bertolino A, Elvevåg B, Callicott JH, Das S, et al. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25(20):5038–45.
Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98(12):6912–22.
Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100(10):6186–91.
Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51(4):593–628.
Gasparini M, Fabrizio E, Bonifati V, Meco G. Cognitive improvement during tolcapone treatment in Parkinson’s disease. J Neural Transm. 1997;104(8–9):887–94.
Farrell SM, Tunbridge EM, Braeutigam S, Harrison PJ. COMT Val(158)Met genotype determines the direction of cognitive effects produced by catechol-O-methyltransferase inhibition. Biol Psychiatry. 2012;71(6):538–534.
Goldman-Rakic P. The cortical dopamine system: role in memory and cognition Adv. Pharmacol. 1998;42:707–11.
Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–5.
McNair DM, Lorr M, Droppleman LF. Revised manual for the profile of mood states. San Diego: Educational and Industrial Testing Service; 1992.
Jorga K, Fotteler B, Heizmann P, Gasser R. Metabolism and excretion of tolcapone, a novel inhibitor of catechol-O-methyltransferase. Br J Clin Pharmacol. 1999;48(4):513–20.
Jorga KM. Pharmacokinetics, pharmacodynamics, and tolerability of tolcapone: a review of early studies in volunteers. Neurology. 1998;50(5 Suppl 5):S31–8.
Friston KJ, Zarahn E, Josephs O, Henson RN, Dale AM. Stochastic designs in event-related fMRI. Neuroimage. 1999;10(5): 607–19.
Cognition and Brain Sciences Unit. DataDiagnostics - MRC CBU Imaging Wiki. [homepage on the Internet]. 2009 [cited 2011 Jun 23]. Available from: Medical Research Council. http://imaging.mrc-cbu.cam.ac.uk/imaging/DataDiagnostics
Oldfield R. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113.
Rasetti R, Mattay VS, Stankevich B, Skjei K, Blasi G, Sambataro F, et al. Modulatory effects of modafinil on neural circuits regulating emotion and cognition. Neuropsychopharmacology. 2010;35(10):2101–9.
Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9(1): 20–26.
Paskavitz JF, Sweet LH, Wellen J, Helmer KG, Rao SM, Cohen RA. Recruitment and stabilization of brain activation within a working memory task; an FMRI study. Brain Imaging Behav. 2010;4(1):5–21.
Spence SA, Green RD, Wilkinson ID, Hunter MD. Modafinil modulates anterior cingulate function in chronic schizophrenia. Br J Psychiatry. 2005;187:55–61.
Hunter MD, Ganesan V, Wilkinson ID, Spence SA. Impact of modafinil on prefrontal executive function in schizophrenia. Am J Psychiatry. 2006;163(12):2184–6.
Acknowledgments
Sophia C. Magalona and Roberta Rasetti contributed equally to the manuscript.
Sophia C. Magalona, Roberta Rasetti, Jingshan Chen, Qiang Chen, Ian Gold, Heather Decot, Joseph H. Callicott, Karen F. Berman, José A. Apud, Daniel R. Weinberger, and Venkata S. Mattay declare that they have no conflict of interest.
This research was supported by direct funding of the Weinberger Lab in the Intramural Research Program of the National Institute of Mental Health, NIH, Bethesda, MD 20892, USA. and the Lieber Institute for Brain Development.
We thank Saumitra Das, MS for his help with data acquisition.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Magalona, S.C., Rasetti, R., Chen, J. et al. Effect of Tolcapone on Brain Activity During a Variable Attentional Control Task: A Double-Blind, Placebo-Controlled, Counter-Balanced Trial in Healthy Volunteers. CNS Drugs 27, 663–673 (2013). https://doi.org/10.1007/s40263-013-0082-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-013-0082-x