Abstract
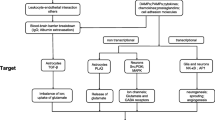
Inflammation is one of the most important endogenous defence mechanisms in an organism. It has been suggested that inflammation plays an important role in the pathophysiology of a number of human epilepsies and convulsive disorders, and there is clinical and experimental evidence to suggest that inflammatory processes within the CNS may either contribute to or be a consequence of epileptogenesis. This review discusses evidence from human studies on the role of inflammation in epilepsy and highlights potential new targets in the inflammatory cascade for antiepileptic drugs. A number of mechanisms have been shown to be involved in CNS inflammatory reactions. These include an inflammatory response at the level of the blood–brain barrier (BBB), immune-mediated damage to the CNS, stress-induced release of inflammatory mediators and direct neuronal dysfunction or damage as a result of inflammatory reactions. Mediators of inflammation in the CNS include interleukin (IL)-1β, tumour necrosis factor-α, nuclear factor-κB and toll-like receptor-4 (TLR4). IL-1β, BBB and high-mobility group box-1-TLR4 signalling appear to be the most promising targets for anticonvulsant agents directed at inflammation. Such agents may provide effective therapy for drug-resistant epilepsies in the future.

Similar content being viewed by others
References
Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3(7):569–81.
Das A, Wallace GC 4th, Holmes C, et al. Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience. 2012;220:237–46.
Sheng JG, Boop FA, Mrak RE, et al. Increased neuronal beta-amyloid precursor protein expression in human temporal lobe epilepsy: association with interleukin-1 alpha immunoreactivity. J Neurochem. 1994;63(5):1872–9.
Bien CG, Urbach H, Schramm J, et al. Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology. 2007;69(12):1236–44.
Crespel A, Coubes P, Rousset MC, et al. Inflammatory reactions in human medial temporal lobe epilepsy with hippocampal sclerosis. Brain Res. 2002;952(2):159–69.
Maldonado M, Baybis M, Newman D, et al. Expression of ICAM-1, TNF-alpha, NF kappa B, and MAP kinase in tubers of the tuberous sclerosis complex. Neurobiol Dis. 2003;14(2):279–90.
Aronica E, Crino PB. Inflammation in epilepsy: clinical observations. Epilepsia. 2011;52(Suppl. 3):26–32.
Bansal SK, Sawhney IM, Chopra JS. Epilepsia partialis continua in Sjogren’s syndrome. Epilepsia. 1987;28(4):362–3.
Cimaz R, Meroni PL, Shoenfeld Y. Epilepsy as part of systemic lupus erythematosus and systemic antiphospholipid syndrome (Hughes syndrome). Lupus. 2006;15(4):191–7.
Russell PW, Haserick JR, Zucker EM. Epilepsy in systemic lupus erythematosus; effect of cortisone and ACTH. AMA Arch Intern Med. 1951;88(1):78–92.
Bovolenta R, Zucchini S, Paradiso B, et al. Hippocampal FGF-2 and BDNF overexpression attenuates epileptogenesis-associated neuroinflammation and reduces spontaneous recurrent seizures. J Neuroinflamm. 2010;7:81.
Vezzani A, French J, Bartfai T, et al. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7(1):31–40.
Musto AE, Samii M. Platelet-activating factor receptor antagonism targets neuroinflammation in experimental epilepsy. Epilepsia. 2011;52(3):551–61.
Marusich E, Louboutin JP, Chekmasova AA, et al. Lymphocyte adhesion to CCR5 ligands is reduced by anti-CCR5 gene delivery. J Neurol Sci. 2011;308(1–2):25–7.
Auvin S, Mazarati A, Shin D, et al. Inflammation enhances epileptogenesis in the developing rat brain. Neurobiol Dis. 2010;40(1):303–10.
Zattoni M, Mura ML, Deprez F, et al. Brain infiltration of leukocytes contributes to the pathophysiology of temporal lobe epilepsy. J Neurosci. 2011;31(11):4037–50.
Ravizza T, Balosso S, Vezzani A. Inflammation and prevention of epileptogenesis. Neurosci Lett. 2011;497(3):223–30.
Pardo CA, Vining EP, Guo L, et al. The pathology of Rasmussen syndrome: stages of cortical involvement and neuropathological studies in 45 hemispherectomies. Epilepsia. 2004;45(5):516–26.
Yang R, Puranam RS, Butler LS, et al. Autoimmunity to munc-18 in Rasmussen’s encephalitis. Neuron. 2000;28(2):375–83.
Bien CG, Bauer J, Deckwerth TL, et al. Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in Rasmussen’s encephalitis. Ann Neurol. 2002;51(3):311–8.
Rogers SW, Andrews PI, Gahring LC, et al. Autoantibodies to glutamate receptor GluR3 in Rasmussen’s encephalitis. Science. 1994;265(5172):648–51.
Wiendl H, Bien CG, Bernasconi P, et al. GluR3 antibodies: prevalence in focal epilepsy but no specificity for Rasmussen’s encephalitis. Neurology. 2001;57(8):1511–4.
Bauer J, Bien CG, Lassmann H. Rasmussen’s encephalitis: a role for autoimmune cytotoxic T lymphocytes. Curr Opin Neurol. 2002;15(2):197–200.
Liu ZS, Wang QW, Wang FL, et al. Serum cytokine levels are altered in patients with West syndrome. Brain Dev. 2001;23(7):548–51.
Hattori H. Spontaneous remission of spasms in West syndrome: implications of viral infection. Brain Dev. 2001;23(7):705–7.
Ravizza T, Boer K, Redeker S, et al. The IL-1beta system in epilepsy-associated malformations of cortical development. Neurobiol Dis. 2006;24(1):128–43.
Peltola J, Palmio J, Korhonen L, et al. Interleukin-6 and interleukin-1 receptor antagonist in cerebrospinal fluid from patients with recent tonic–clonic seizures. Epilepsy Res. 2000;41(3):205–11.
Peltola J, Laaksonen J, Haapala AM, et al. Indicators of inflammation after recent tonic–clonic epileptic seizures correlate with plasma interleukin-6 levels. Seizure. 2002;11(1):44–6.
Hulkkonen J, Koskikallio E, Rainesalo S, et al. The balance of inhibitory and excitatory cytokines is differently regulated in vivo and in vitro among therapy resistant epilepsy patients. Epilepsy Res. 2004;59(2–3):199–205.
Lehtimaki KA, Liimatainen S, Peltola J, et al. The serum level of interleukin-6 in patients with intellectual disability and refractory epilepsy. Epilepsy Res. 2011;95(1–2):184–7.
Haspolat S, Mihci E, Coskun M, et al. Interleukin-1beta, tumor necrosis factor-alpha, and nitrite levels in febrile seizures. J Child Neurol. 2002;17(10):749–51.
Tutuncuoglu S, Kutukculer N, Kepe L, et al. Proinflammatory cytokines, prostaglandins and zinc in febrile convulsions. Pediatr Int. 2001;43(3):235–9.
Virta M, Hurme M, Helminen M. Increased plasma levels of pro- and anti-inflammatory cytokines in patients with febrile seizures. Epilepsia. 2002;43(8):920–3.
Ichiyama T, Nishikawa M, Yoshitomi T, et al. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 in cerebrospinal fluid from children with prolonged febrile seizures: comparison with acute encephalitis/encephalopathy. Neurology. 1998;50(2):407–11.
Lahat E, Livne M, Barr J, et al. Interleukin-1beta levels in serum and cerebrospinal fluid of children with febrile seizures. Pediatr Neurol. 1997;17(1):34–6.
Miceli Sopo S, Cuomo B, Federico G, et al. In vivo and in vitro production of interleukin-1 after febrile convulsions. Pediatr Med Chir. 2001;23(2):83–7.
Gultekin SH, Rosenfeld MR, Voltz R, et al. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123(Pt 7):1481–94.
Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127(Pt 3):701–12.
Tan KM, Lennon VA, Klein CJ, et al. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology. 2008;70(20):1883–90.
Saiz A, Blanco Y, Sabater L, et al. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131(Pt 10):2553–63.
Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-d-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25–36.
Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9(1):67–76.
Riikonen R. Infantile spasms: therapy and outcome. J Child Neurol. 2004;19(6):401–4.
Dravet C, Natale O, Magaudda A, et al. Status epilepticus in the Lennox-Gastaut syndrome. Rev Electroencephalogr Neurophysiol Clin. 1986;15(4):361–8.
van Engelen BG, Renier WO, Weemaes CM, et al. High-dose intravenous immunoglobulin treatment in cryptogenic West and Lennox–Gastaut syndrome; an add-on study. Eur J Pediatr. 1994;153(10):762–9.
Mikati MA, Saab R, Fayad MN, et al. Efficacy of intravenous immunoglobulin in Landau–Kleffner syndrome. Pediatr Neurol. 2002;26(4):298–300.
Granata T, Fusco L, Gobbi G, et al. Experience with immunomodulatory treatments in Rasmussen’s encephalitis. Neurology. 2003;61(12):1807–10.
Marchi N, Granata T, Freri E, et al. Efficacy of anti-inflammatory therapy in a model of acute seizures and in a population of pediatric drug resistant epileptics. PLoS One. 2011;6(3):e18200.
Vezzani A, Conti M, De Luigi A, et al. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci. 1999;19(12):5054–65.
Kanemoto K, Kawasaki J, Miyamoto T, et al. Interleukin (IL)1beta, IL-1alpha, and IL-1 receptor antagonist gene polymorphisms in patients with temporal lobe epilepsy. Ann Neurol. 2000;47(5):571–4.
Peltola J, Keranen T, Rainesalo S, et al. Polymorphism of the interleukin-1 gene complex in localization-related epilepsy. Ann Neurol. 2001;50(2):275–6.
Buono RJ, Ferraro TN, O’Connor MJ, et al. Lack of association between an interleukin 1 beta (IL-1beta) gene variation and refractory temporal lobe epilepsy. Epilepsia. 2001;42(6):782–4.
Heils A, Haug K, Kunz WS, et al. Interleukin-1beta gene polymorphism and susceptibility to temporal lobe epilepsy with hippocampal sclerosis. Ann Neurol. 2000;48(6):948–50.
Boer K, Crino PB, Gorter JA, et al. Gene expression analysis of tuberous sclerosis complex cortical tubers reveals increased expression of adhesion and inflammatory factors. Brain Pathol. 2010;20(4):704–19.
van Gassen KLI, de Wit M, Koerkamp MJAG, et al. Possible role of the innate immunity in temporal lobe epilepsy. Epilepsia. 2008;49(6):1055–65.
Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46(11):1724–43.
Gallagher BB, Flanigin HF, King DW, et al. The effect of electrical stimulation of medial temporal lobe structures in epileptic patients upon ACTH, prolactin, and growth hormone. Neurology. 1987;37(2):299–303.
Galimberti CA, Magri F, Copello F, et al. Seizure frequency and cortisol and dehydroepiandrosterone sulfate (DHEAS) levels in women with epilepsy receiving antiepileptic drug treatment. Epilepsia. 2005;46(4):517–23.
Calabrese VP, Gruemer HD, Tripathi HL, et al. Serum cortisol and cerebrospinal fluid beta-endorphins in status epilepticus: their possible relation to prognosis. Arch Neurol. 1993;50(7):689–93.
Yang T, Zhou D, Stefan H. Why mesial temporal lobe epilepsy with hippocampal sclerosis is progressive: uncontrolled inflammation drives disease progression? J Neurol Sci. 2010;296(1–2):1–6.
Amato C, Elia M, Musumeci SA, et al. Transient MRI abnormalities associated with partial status epilepticus: a case report. Eur J Radiol. 2001;38(1):50–4.
Lansberg MG, O’Brien MW, Norbash AM, et al. MRI abnormalities associated with partial status epilepticus. Neurology. 1999;52(5):1021–7.
Paladin F, Bonazza A, Mameli R, et al. Cryptogenic temporal lobe epilepsy: semi-quantitative interictal 99mTc HMPAO SPECT: statistical correlation with clinical data and EEG. Ital J Neurol Sci. 1999;20(4):237–42.
Pavlovsky L, Seiffert E, Heinemann U, et al. Persistent BBB disruption may underlie alpha interferon-induced seizures. J Neurol. 2005;252(1):42–6.
Tomkins O, Kaufer D, Korn A, et al. Frequent blood-brain barrier disruption in the human cerebral cortex. Cell Mol Neurobiol. 2001;21(6):675–91.
Pachter JS, de Vries HE, Fabry Z. The blood-brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol. 2003;62(6):593–604.
Librizzi L, Noe F, Vezzani A, et al. Seizure-induced brain-borne inflammation sustains seizure recurrence and blood–brain barrier damage. Ann Neurol. 2012;72(1):82–90.
Zucker DK, Wooten GF, Lothman EW. Blood–brain barrier changes with kainic acid-induced limbic seizures. Exp Neurol. 1983;79(2):422–33.
Fabene PF, Navarro G, Martinello M, et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nature Med. 2008;14(12):1377–83.
Duffy BA, Choy M, Riegler J, et al. Imaging seizure-induced inflammation using an antibody targeted iron oxide contrast agent. Neuroimage. 2012;60(2):1149–55.
Louboutin JP, Chekmasova A, Marusich E, et al. Role of CCR5 and its ligands in the control of vascular inflammation and leukocyte recruitment required for acute excitotoxic seizure induction and neural damage. FASEB J. 2011;25(2):737–53.
Tomkins O, Friedman O, Ivens S, et al. Blood–brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. Neurobiol Dis. 2007;25(2):367–77.
Uva L, Librizzi L, Marchi N, et al. Acute induction of epileptiform discharges by pilocarpine in the in vitro isolated guinea-pig brain requires enhancement of blood–brain barrier permeability. Neuroscience. 2008;151(1):303–12.
Friedman A, Dingledine R. Molecular cascades that mediate the influence of inflammation on epilepsy. Epilepsia. 2011;52(Suppl. 3):33–9.
Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21(11):471–6.
Huerta PT, Kowal C, DeGiorgio LA, et al. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci USA. 2006;103(3):678–83.
Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. 1999;10(9):359–68.
Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2(10):734–44.
Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3(3):216–27.
Stoll G, Jander S, Schroeter M. Cytokines in CNS disorders: neurotoxicity versus neuroprotection. J Neural Transm Suppl. 2000;59:81–9.
Graus F, Saiz A, Dalmau J. Antibodies and neuronal autoimmune disorders of the CNS. J Neurol. 2009;257(4):509–17.
Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091–8.
Liou HH, Wang CR, Chou HC, et al. Anticardiolipin antisera from lupus patients with seizures reduce a GABA receptor-mediated chloride current in snail neurons. Life Sci. 1994;54(15):1119–25.
Wyss-Coray T, Lin C, Yan F, et al. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7(5):612–8.
Aloisi F. Immune function of microglia. Glia. 2001;36(2):165–79.
Vezzani A, Maroso M, Balosso S, et al. IL-1 receptor/toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun. 2011;25(7):1281–9.
Becher B, Prat A, Antel JP. Brain–immune connection: immuno-regulatory properties of CNS-resident cells. Glia. 2000;29(4):293–304.
Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76(2):77–98.
Rizzi M, Perego C, Aliprandi M, et al. Glia activation and cytokine increase in rat hippocampus by kainic acid-induced status epilepticus during postnatal development. Neurobiol Dis. 2003;14(3):494–503.
Turrin NP, Rivest S. Innate immune reaction in response to seizures: implications for the neuropathology associated with epilepsy. Neurobiol Dis. 2004;16(2):321–34.
Plata-Salaman CR, Ilyin SE, Turrin NP, et al. Kindling modulates the IL-1beta system, TNF-alpha, TGF-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Mol Brain Res. 2000;75(2):248–58.
De Simoni MG, Perego C, Ravizza T, et al. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci. 2000;12(7):2623–33.
Vezzani A, Moneta D, Conti M, et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci USA. 2000;97(21):11534–9.
Dube C, Vezzani A, Behrens M, et al. Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol. 2005;57(1):152–5.
Yuhas Y, Shulman L, Weizman A, et al. Involvement of tumor necrosis factor alpha and interleukin-1beta in enhancement of pentylenetetrazole-induced seizures caused by Shigella dysenteriae. Infect Immun. 1999;67(3):1455–60.
Ravizza T, Lucas SM, Balosso S, et al. Inactivation of caspase-1 in rodent brain: a novel anticonvulsive strategy. Epilepsia. 2006;47(7):1160–8.
Vezzani A, Baram TZ. New roles for interleukin-1 beta in the mechanisms of epilepsy. Epilepsy Curr. 2007;7(2):45–50.
Probert L, Akassoglou K, Pasparakis M, et al. Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factor alpha. Proc Natl Acad Sci USA. 1995;92(24):11294–8.
Meli DN, Loeffler JM, Baumann P, et al. In pneumococcal meningitis a novel water-soluble inhibitor of matrix metalloproteinases and TNF-alpha converting enzyme attenuates seizures and injury of the cerebral cortex. J Neuroimmunol. 2004;151(1–2):6–11.
Yuhas Y, Weizman A, Ashkenazi S. Bidirectional concentration-dependent effects of tumor necrosis factor alpha in Shigella dysenteriae-related seizures. Infect Immun. 2003;71(4):2288–91.
Balosso S, Ravizza T, Perego C, et al. Tumor necrosis factor-alpha inhibits seizures in mice via p75 receptors. Ann Neurol. 2005;57(6):804–12.
Ravizza T, Vezzani A. Status epilepticus induces time-dependent neuronal and astrocytic expression of interleukin-1 receptor type I in the rat limbic system. Neuroscience. 2006;137(1):301–8.
De Sarro G, Rotiroti D, Audino MG, et al. Effects of interleukin-2 on various models of experimental epilepsy in DBA/2 mice. Neuroimmunomodulation. 1994;1(6):361–9.
Nistico G, De Sarro G. Behavioral and electrocortical spectrum power effects after microinfusion of lymphokines in several areas of the rat brain. Ann N Y Acad Sci. 1991;621:119–34.
Cuevas P, Gimenez-Gallego G. Antiepileptic effects of acidic fibroblast growth factor examined in kainic acid-mediated seizures in the rat. Neurosci Lett. 1996;203(1):66–8.
Liu Z, Holmes GL. Basic fibroblast growth factor is highly neuroprotective against seizure-induced long-term behavioural deficits. Neuroscience. 1997;76(4):1129–38.
Liu Z, Holmes GL. Basic fibroblast growth factor-induced seizures in rats. Neurosci Lett. 1997;233(2–3):85–8.
Campbell IL, Abraham CR, Masliah E, et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci USA. 1993;90(21):10061–5.
Cunningham AJ, Murray CA, O’Neill LA, et al. Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203(1):17–20.
Samland H, Huitron-Resendiz S, Masliah E, et al. Profound increase in sensitivity to glutamatergic- but not cholinergic agonist-induced seizures in transgenic mice with astrocyte production of IL-6. J Neurosci Res. 2003;73(2):176–87.
Lehtimaki KA, Peltola J, Koskikallio E, et al. Expression of cytokines and cytokine receptors in the rat brain after kainic acid-induced seizures. Brain Res Mol Brain Res. 2003;110(2):253–60.
Adams J, Collaco-Moraes Y, de Belleroche J. Cyclooxygenase-2 induction in cerebral cortex: an intracellular response to synaptic excitation. J Neurochem. 1996;66(1):6–13.
Kamikawa H, Hori T, Nakane H, et al. IL-1beta increases norepinephrine level in rat frontal cortex: involvement of prostanoids, NO, and glutamate. Am J Physiol. 1998;275(3 Pt 2):R803–10.
Schneider H, Pitossi F, Balschun D, et al. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci USA. 1998;95(13):7778–83.
Viviani B, Bartesaghi S, Gardoni F, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23(25):8692–700.
Wang S, Cheng Q, Malik S, et al. Interleukin-1beta inhibits gamma-aminobutyric acid type A (GABA(A)) receptor current in cultured hippocampal neurons. J Pharmacol Exp Ther. 2000;292(2):497–504.
Ye ZC, Sontheimer H. Cytokine modulation of glial glutamate uptake: a possible involvement of nitric oxide. Neuroreport. 1996;7(13):2181–5.
Ravizza T, Noe F, Zardoni D, et al. Interleukin converting enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1beta production. Neurobiol Dis. 2008;31(3):327–33.
Vezzani A, Moneta D, Richichi C, et al. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43(Suppl. 5):30–5.
Heida JG, Pittman QJ. Causal links between brain cytokines and experimental febrile convulsions in the rat. Epilepsia. 2005;46(12):1906–13.
Marchi N, Fan Q, Ghosh C, et al. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis. 2009;33(2):171–81.
Bauer J, Berkenbosch F, Van Dam AM, et al. Demonstration of interleukin-1 beta in Lewis rat brain during experimental allergic encephalomyelitis by immunocytochemistry at the light and ultrastructural level. J Neuroimmunol. 1993;48(1):13–21.
Griffin WS, Stanley LC, Ling C, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86(19):7611–5.
Rijkers K, Majoie HJ, Hoogland G, et al. The role of interleukin-1 in seizures and epilepsy: a critical review. Exp Neurol. 2009;216(2):258–71.
Docagne F, Campbell SJ, Bristow AF, et al. Differential regulation of type I and type II interleukin-1 receptors in focal brain inflammation. Eur J Neurosci. 2005;21(5):1205–14.
Jung KH, Chu K, Lee ST, et al. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis. 2006;23(2):237–46.
Kim HJ, Chung JI, Lee SH, et al. Involvement of endogenous prostaglandin F2alpha on kainic acid-induced seizure activity through FP receptor: the mechanism of proconvulsant effects of COX-2 inhibitors. Brain Res. 2008;1193:153–61.
Kunz T, Oliw EH. The selective cyclooxygenase-2 inhibitor rofecoxib reduces kainate-induced cell death in the rat hippocampus. Eur J Neurosci. 2001;13(3):569–75.
Claycomb RJ, Hewett SJ, Hewett JA. Prophylactic, prandial rofecoxib treatment lacks efficacy against acute PTZ-induced seizure generation and kindling acquisition. Epilepsia. 2011;52(2):273–83.
Naffah-Mazzacoratti MG, Bellissimo MI, Cavalheiro EA. Profile of prostaglandin levels in the rat hippocampus in pilocarpine model of epilepsy. Neurochem Int. 1995;27(6):461–6.
Oliveira MS, Furian AF, Rambo LM, et al. Modulation of pentylenetetrazol-induced seizures by prostaglandin E2 receptors. Neuroscience. 2008;152(4):1110–8.
Holtman L, van Vliet EA, van Schaik R, et al. Effects of SC58236, a selective COX-2 inhibitor, on epileptogenesis and spontaneous seizures in a rat model for temporal lobe epilepsy. Epilepsy Res. 2009;84(1):56–66.
Galic MA, Riazi K, Heida JG, et al. Postnatal inflammation increases seizure susceptibility in adult rats. J Neurosci. 2008;28(27):6904–13.
Kawai T, Akira S. Signaling to NF-kappaB by toll-like receptors. Trends Mol Med. 2007;13(11):460–9.
Bianchi ME, Manfredi AA. Immunology: dangers in and out. Science. 2009;323(5922):1683–4.
Maroso M, Balosso S, Ravizza T, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16(4):413–9.
Ito H, Mori K, Toda Y, et al. A case of acute encephalitis with refractory, repetitive partial seizures, presenting autoantibody to glutamate receptor Gluepsilon2. Brain Dev. 2005;27(7):531–4.
Takahashi Y, Mori H, Mishina M, et al. Autoantibodies to NMDA receptor in patients with chronic forms of epilepsia partialis continua. Neurology. 2003;61(7):891–6.
Acknowledgments
The authors thank Iain Patefield and Melanie Gatt who provided medical writing assistance, and Andrea Bothwell who provided post-submission amendments, all of inScience Communications, Springer Healthcare. This assistance was funded by UCB Pharma. Mercé Falip has received funding for lectures from the Medical Department of UCB; Xavier Salas-Puig declares no conflict of interest. Carlos Cara is an employee of UCB Pharma.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Falip, M., Salas-Puig, X. & Cara, C. Causes of CNS Inflammation and Potential Targets for Anticonvulsants. CNS Drugs 27, 611–623 (2013). https://doi.org/10.1007/s40263-013-0078-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-013-0078-6