Abstract
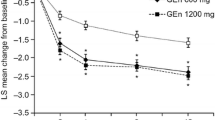
Oral gabapentin enacarbil is approved in adult patients for the treatment of moderate to severe primary restless legs syndrome (RLS) [featured indication] and the management of postherpetic neuralgia. In the 12-week Patient Improvements in Vital Outcomes following Treatment (PIVOT) RLS I and II trials in adult patients with moderate to severe primary RLS (n > 500 total evaluable), once-daily gabapentin enacarbil 600 or 1,200 mg significantly improved mean International Restless Legs Scale (IRLS) total scores compared with placebo, with significantly higher investigator-rated Clinical Global Impression-Improvement (CGI-I) responder rates in gabapentin enacarbil groups than in placebo groups. Improvements in other sleep outcomes (assessed using various scales) also generally favoured gabapentin enacarbil treatment. These data are supported by results from a polysomnography, crossover (two 4-week treatment periods) trial (n > 100 evaluable). Improvements in RLS symptoms with gabapentin enacarbil were maintained in a 52-week extension study of clinical trials, including PIVOT RLS I and II. The longer-term efficacy of gabapentin enacarbil in patients with moderate to severe RLS was also demonstrated in the 36-week PIVOT RLS Maintenance study and a 52-week noncomparative study conducted in Japan. Gabapentin enacarbil was generally well tolerated in adult patients with RLS participating in short- and longer-term clinical trials. The most common treatment-emergent adverse events were somnolence/sedation and dizziness. Most adverse events were of mild to moderate severity, with relatively few patients discontinuing treatment because of an adverse event.


Similar content being viewed by others
References
Leschziner G, Gringras P. Restless legs syndrome. BMJ. 2012;344:e3056.
Yaltho TC, Ondo WG. The use of gabapentin enacarbil in the treatment of restless legs syndrome. Ther Adv Neurol Disord. 2010;3(5):269–75.
Satija P, Ondo WG. Restless legs syndrome: pathophysiology, diagnosis and treatment. CNS Drugs. 2008;22(6):497–518.
Sivam S, Yee BJ. Role of gabapentin enacarbil XR in restless legs syndrome. Ther Clin Risk Manag. 2012;8:201–8.
National Institute of Neurological Disorders and Stroke. Restless legs syndrome fact sheet [online]. Available from URL: http://www.ninds.nih.gov/disorders/restless_legs/detail_restless_legs.htm. Accessed 15 Oct 2012.
Cundy KC, Branch R, Chernov-Rogan T, et al. XP13512 [(±)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: I. Design, synthesis, enzymatic conversion and transport by intestinal solute transporters. J Pharmacol Exp Ther. 2004;311(1):315–23.
GlaxoSmithKline, XenoPort Inc. Horizant (gabapentin enacarbil) extended-release tablets for oral use: US prescribing information [online]. Available from URL: http://us.gsk.com/products/assets/us_horizant.pdf. Accessed 23 Aug 2012.
Lal R, Sukbuntherng J, Luo W, et al. Population pharmacokinetics and pharmacodynamics of gabapentin after administration of gabapentin enacarbil. J Clin Pharmacol. 2012. doi:10.1177/0091270012439209.
Chen D, Lal R, Zomorodi K, et al. Evaluation of gabapentin enacarbil on cardiac repolarization: a randomized, double-blind, placebo- and active-controlled, crossover thorough QT/QTc study in healthy adults. Clin Ther. 2012;34(351–62):e3.
Lal R, Sukbuntherng J, Luo W, et al. Pharmacokinetics and tolerability of single escalating doses of gabapentin enacarbil: a randomized-sequence, double-blind, placebo-controlled crossover study in healthy volunteers. Clin Ther. 2009;31(8):1776–86.
Cundy KC, Annamalai T, Bu L, et al. XP13512 [(±)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: II. Improved oral bioavailability, dose proportionality, and colonic absorption compared with gabapentin in rats and monkeys. J Pharmacol Exp Ther. 2004;311(1):324–33.
Lal R, Ellenbogen A, Chen D, et al. A randomized, double-blind, placebo-controlled, dose-response study to assess the pharmacokinetics, efficacy, and safety of gabapentin enacarbil in subjects with restless legs syndrome. Clin Neuropharmacol. 2012;35(4):165–73.
Cundy KC, Sastry S, Luo WD, et al. Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin. J Clin Pharmacol. 2008;48(12):1378–88.
Lal R, Sukbuntherng J, Luo W, et al. The effect of food with varying fat content on the clinical pharmacokinetics of gabapentin after oral administration of gabapentin enacarbil. Int J Clin Pharmacol Ther. 2010;48(2):120–8.
Lal R, Sukbuntherng J, Ho J, et al. A phase I, single-dose study of the disposition of 14C-radiolabeled gabapentin enacarbil in healthy male volunteers. Int J Clin Pharmacol Ther. 2011;49(2):109–15.
Lal R, Sukbuntherng J, Luo W, et al. Clinical pharmacokinetics of gabapentin after administration of gabapentin enacarbil extended-release tablets in patients with varying degrees of renal function using data from an open-label, single-dose pharmacokinetic study. Clin Ther. 2012;34(1):201–13.
Lal R, Sukbuntherng J, Luo W, et al. Clinical pharmacokinetic drug interaction studies of gabapentin enacarbil, a novel transported prodrug of gabapentin, with naproxen and cimetidine. Br J Clin Pharmacol. 2010;69(5):498–507.
Kushida CA, Becker PM, Ellenbogen AL, et al. Randomized, double-blind, placebo-controlled study of XP13512/GSK1838262 in patients with RLS. Neurology. 2009;72(5):439–46.
Lee DO, Ziman RB, Perkins AT, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med. 2011;7(3):282–92.
Winkelman JW, Bogan RK, Schmidt MH, et al. Randomized polysomnography study of gabapentin enacarbil in subjects with restless legs syndrome. Mov Disord. 2011;26(11):2065–72.
Bogan R, Ellenbogen AL, Becker PM, et al. Gabapentin enacarbil improves RLS symptoms and subjective measures of sleep in subjects with primary restless legs syndrome with and without severe sleep disturbance: secondary analyses from two studies [abstract no. P02.283]. In: 62nd Annual Meeting of the American Academy of Neurology; 2010 April 10–17; Toronto.
VanMeter SA, Kavanagh ST, Warren S, et al. Dose response of gabapentin enacarbil versus placebo in subjects with moderate-to-severe primary restless legs syndrome: an integrated analysis of three 12-week studies. CNS Drugs. 2012;26(9):773–80.
Canafax DM, Bhanegaonkar A, Bharmal M, et al. Validation of the post sleep questionnaire for assessing subjects with restless legs syndrome: results from two double-blind, multicenter, placebo-controlled clinical trials. BMC Neurol. 2011;11:48.
Lee D, Ziman R, Perkins A, et al. Gabapentin enacarbil improves pain associated with restless legs syndrome (RLS) [abstract no. 311]. J Pain. 2010;11(4 Suppl. 1):S53.
Bogan RK, Bornemann MAC, Kushida CA, et al. Long-term maintenance treatment of restless legs syndrome with gabapentin enacarbil: a randomized controlled study. [Erratum appears in Mayo Clin Proc. 2010, 85(7), pp. 693–4]. Mayo Clin Proc. 2010;85(6):512–21.
Ellenbogen AL, Thein SG, Winslow DH, et al. A 52-week study of gabapentin enacarbil in restless legs syndrome. Clin Neuropharmacol. 2011;34(1):8–16.
Inoue Y, Uchimura N, Kuroda K, et al. Long-term efficacy and safety of gabapentin enacarbil in Japanese restless legs syndrome patients. Prog Neuro Psychopharmacol Biol Psychiatry. 2012;36(2):251–7.
Irizarry MC, Webb DJ, Boudiaf N, et al. Risk of cancer in patients exposed to gabapentin in two electronic medical record systems. Pharmacoepidem Drug Safety. 2012;21:214–25.
Astellas Pharma Inc., XenoPort Inc. Astellas and XenoPort announce the launch of Regnite® tablets for restless legs syndrome in Japan [online]. Available from URL: http://www.astellas.co/en/corporate/news/pdf/120709.eg.pdf Accessed 23 Aug 2012.
Aurora RN, Kristo DA, Bista SR, et al. The treatment of restless legs syndrome and periodic limb movement disorders in adults: an update for 2012. Practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep. 2012;35(8):1039–62.
International Restless Legs Syndrome Study Group Task Force. Summary of recommendations for the long-term treatment of RLS/WED from an IRLSSG task force [online]. Available from URL: http://irlssg.org/wp-content/uploads/2012/07/Summary-of-RLS-treatment-recommendations-FINAL.pdf. Accessed 15 Oct 2012.
Vignatelli L, Billiard M, Clarenbach P, et al. EFNS guidelines on management of restless legs syndrome and periodic limb movement disorder in sleep. Eur J Neurol. 2006;13(10):1049–65.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: J. Chen, Lorna Linda University Movement Disorders Center, Schools of Medicine and Pharmacy, Lorna Linda, California, USA; C. Guilleminault, Stanford University Sleep Medicine Division, Redwood, California, USA; H.B. Lee, John Hopkins University School of Medicine, Department of Psychiatry and Behavioral Sciences, Baltimore, Maryland, USA.
Rights and permissions
About this article
Cite this article
Scott, L.J. Gabapentin Enacarbil. CNS Drugs 26, 1073–1083 (2012). https://doi.org/10.1007/s40263-012-0020-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-012-0020-3