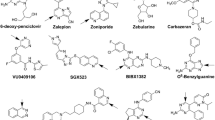
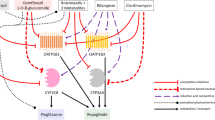
Abstract
Background and Objective
Early investigations into drug–drug interactions (DDIs) involving cytochrome P450 2C8 (CYP2C8) have highlighted the complexity of interactions between CYP2C8 substrate drugs, including montelukast, desloratadine, pioglitazone, repaglinide, and cerivastatin (the latter two being OATP1B1 substrates), and standardized CYP2C8 inhibitors such as clopidogrel (Clop) and gemfibrozil (Gem). These interactions have proven challenging to predict based solely on simple CYP inhibition. A hypothesis has emerged suggesting that these substrate drugs first distribute to UDP-glucuronosyltransferase (UGT) before undergoing oxidation by CYP2C8, resulting in bidirectional elimination. The process of drug distribution to UGT is believed to significantly impact these DDIs. This study aims to explore the intricate interplay between UGT and CYP2C8 in the context of DDIs involving CYP2C8 substrates affected by Clop and Gem.
Methods
Plasma-level data for the unchanged drug and its metabolite, drawn from the respective literature, formed the basis of our analysis. We evaluated the enzymatic inhibitory activities of DDIs and utilized simulations to estimate plasma levels of the unchanged victim drug and its metabolite in each DDI. This was accomplished by employing a functional relationship that considered the fractional contributions of CYP2C8 and UGT to clearance, perpetrator-specific inhibitory activities against CYP2C8, and drug distribution to UGT.
Results
Our findings emphasize the pivotal role of UGT-mediated distribution in the context of CYP2C8 substrate metabolism, particularly in the complex DDIs induced by Clop and Gem. In these DDIs, Gem exerts inhibitory effects on both UGT and CYP2C8, whereas Clop (specifically its metabolite, Clop-COOH) solely targets CYP2C8. Importantly, the inhibition of CYP2C8 by both Clop and Gem is achieved through a non-competitive mechanism, driven by the actions of their acyl-glucuronides. Clop and Gem exhibit inhibition activities accounting for 85% (pAi,CYP2C8 = 7) and 93% (pAi,CYP2C8 = 15), respectively. In contrast, Gem's inhibition of UGT is relatively modest (50%, pAi,UGT(d) = 2), and it operates through a non-specific, competitive process in drug distribution to UGT. Within this context, our UGT-CYP2C8 interplay model offers an accurate means of predicting the alterations resulting from DDIs, encompassing changes in plasma levels of the unchanged drug and its metabolites, as well as shifts in metabolite formation rates. Our analysis highlights the critical importance of considering the fractional contributions of CYP2C8 and UGT to the victim drug's clearance (fm,CYP2C8; fm,UGT) in DDI prediction. Furthermore, our examination of DDIs involving OATP1B1 substrate drugs underscores that accounting for the hepatic uptake transporters' role in the liver is superfluous in DDI prediction.
Conclusion
These findings substantially enhance our comprehension of CYP2C8-mediated oxidation and DDIs, holding crucial implications for drug development and the planning of clinical trials involving these inhibitors.





Similar content being viewed by others
References
Okuda H, Nishiyama T, Ogura K, Nagayama S, Ikeda K, Yamaguchi S, Nakamura Y, Kawaguchi Y, Watabe T. Lethal drug interactions of sorivudine, a new antiviral drug, with oral 5-fluorouracil prodrugs. Drug Metab Dispos. 1997;25(5):270–3.
Iga K. Use of three-compartment physiologically based pharmacokinetic modeling to predict hepatic blood levels of fluvoxamine relevant for drug-drug interactions. J Pharm Sci. 2015;104(4):1478–91.
Iga K. Simulation of metabolic drug-drug interactions perpetrated by fluvoxamine using hybridized two-compartment hepatic drug-pool-based tube modeling and estimation of in vivo inhibition constants. J Pharm Sci. 2015;104(10):3565–77.
Iga K. Dynamic and static simulations of fluvoxamine-perpetrated drug-drug interactions using multiple cytochrome P450 inhibition modeling, and determination of perpetrator-specific CYP isoform inhibition constants and fractional CYP isoform contributions to victim clearance. J Pharm Sci. 2016;105(3):1307–17.
Iga K, Kiriyama A. Simulations of cytochrome P450 3A4-mediated drug-drug interactions by simple two-compartment model-assisted static method. J Pharm Sci. 2017;106(5):1426–38.
Iga K, Kiriyama A. Usefulness of two-compartment model-assisted and static overall inhibitory-activity method for prediction of drug-drug interaction. Biol Pharm Bull. 2017;40(12):2024–37.
Backman JT, Filppula AM, Niemi M, Neuvonen PJ. Role of cytochrome P450 2C8 in drug metabolism and interactions. Pharmacol Rev. 2016;68(1):168–241.
Karonen T, Neuvonen PJ, Backman JT. CYP2C8 but not CYP3A4 is important in the pharmacokinetics of montelukast. Br J Clin Pharmacol. 2012;73(2):257–67.
Itkonen MK, Tornio A, Filppula AM, Neuvonen M, Neuvonen PJ, Niemi M, Backman JT. Clopidogrel but not prasugrel significantly inhibits the CYP2C8-mediated metabolism of montelukast in humans. Clin Pharmacol Ther. 2018;104(3):495–504.
Itkonen MK, Tornio A, Neuvonen M, Neuvonen PJ, Niemi M, Backman JT. Clopidogrel and gemfibrozil strongly inhibit the CYP2C8-dependent formation of 3-hydroxydesloratadine and increase desloratadine exposure in humans. Drug Metab Dispos. 2019;47(4):377–85.
Niemi M, Backman JT, Neuvonen M, Neuvonen PJ. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics and pharmacodynamics of repaglinide: potentially hazardous interaction between gemfibrozil and repaglinide. Diabetologia. 2003;46(3):347–51.
Ogilvie BW, Zhang D, Li W, Rodrigues AD, Gipson AE, Holsapple J, Toren P, Parkinson A. Glucuronidation converts gemfibrozil to a potent, metabolism-dependent inhibitor of CYP2C8: implications for drug-drug interactions. Drug Metab Dispos. 2006;34(1):191–7.
Backman JT, Honkalammi J, Neuvonen M, Kurkinen KJ, Tornio A, Niemi M, Neuvonen PJ. CYP2C8 activity recovers within 96 hours after gemfibrozil dosing: estimation of CYP2C8 half-life using repaglinide as an in vivo probe. Drug Metab Dispos. 2009;37(12):2359–66.
Tornio A, Filppula AM, Kailari O, Neuvonen M, Nyrönen TH, Tapaninen T, Neuvonen PJ, Niemi M, Backman JT. Glucuronidation converts clopidogrel to a strong time-dependent inhibitor of CYP2C8: a phase II metabolite as a perpetrator of drug-drug interactions. Clin Pharmacol Ther. 2014;96(4):498–507.
Kahma H, Filppula AM, Neuvonen M, Tarkiainen EK, Tornio A, Holmberg MT, Itkonen MK, Finel M, Neuvonen PJ, Niemi M, Backman JT. Clopidogrel carboxylic acid glucuronidation is mediated mainly by UGT2B7, UGT2B4, and UGT2B17: implications for pharmacogenetics and drug-drug interactions. Drug Metab Dispos. 2018;46(2):141–50.
Takagi M, Sakamoto M, Itoh T, Fujiwara R. Underlying mechanism of drug-drug interaction between pioglitazone and gemfibrozil: gemfibrozil acyl-glucuronide is a mechanism-based inhibitor of CYP2C8. Drug Metab Pharmacokinet. 2015;30(4):288–94.
Honkalammi J, Niemi M, Neuvonen PJ, Backman JT. Mechanism-based inactivation of CYP2C8 by gemfibrozil occurs rapidly in humans. Clin Pharmacol Ther. 2011;89(4):579–86.
Shah MB. Inhibition of CYP2C8 by acyl glucuronides of gemfibrozil and clopidogrel: pharmacological significance, progress and challenges. Biomolecules. 2022;12(9):1218.
Tornio A, Neuvonen PJ, Niemi M, Backman JT. Role of gemfibrozil as an inhibitor of CYP2C8 and membrane transporters. Expert Opin Drug Metab Toxicol. 2017;13(1):83–95.
Nishihara M, Sudo M, Kamiguchi H, Kawaguchi N, Maeshiba Y, Kiyota Y, Takahashi J, Tagawa Y, Kondo T, Asahi S. Metabolic fate of sipoglitazar, a novel oral PPAR agonist with activities for PPAR-γ, -α and -δ, in rats and monkeys and comparison with humans in vitro. Drug Metab Pharmacokinet. 2012;27(2):223–31.
Nishihara M, Sudo M, Kawaguchi N, Takahashi J, Kiyota Y, Kondo T, Asahi S. An unusual metabolic pathway of sipoglitazar, a novel antidiabetic agent: cytochrome P450-catalyzed oxidation of sipoglitazar acyl glucuronide. Drug Metab Dispos. 2012;40(2):249–58.
Stringer F, Scott G, Valbuena M, Kinley J, Nishihara M, Urquhart R. The effect of genetic polymorphisms in UGT2B15 on the pharmacokinetic profile of sipoglitazar, a novel anti-diabetic agent. Eur J Clin Pharmacol. 2013;69(3):423–30.
Kazmi F, Barbara JE, Yerino P, Parkinson A. A long-standing mystery solved: the formation of 3-hydroxydesloratadine is catalyzed by CYP2C8 but prior glucuronidation of desloratadine by UDP-glucuronosyltransferase 2B10 is an obligatory requirement. Drug Metab Dispos. 2015;43(4):523–33.
Kazmi F, Yerino P, Barbara JE, Parkinson A. Further characterization of the metabolism of desloratadine and its cytochrome P450 and UDP-glucuronosyltransferase inhibition potential: identification of desloratadine as a relatively selective UGT2B10 inhibitor. Drug Metab Dispos. 2015;43:1294–302.
Gan J, Chen W, Shen H, Gao L, Hong Y, Tian Y, Li W, Zhang Y, Tang Y, Zhang H, Humphreys WG, Rodrigues AD. Repaglinide-gemfibrozil drug interaction: inhibition of repaglinide glucuronidation as a potential additional contributing mechanism. Br J Clin Pharmacol. 2010;70(6):870–80.
VandenbBrink BM, Foti RS, Rock DA, Wienkers LC, Wahlstrom JL. Evaluation of CYP2C8 inhibition in vitro: utility of montelukast as a selective CYP2C8 probe substrate. Drug Metab Dispos. 2011;39(9):1546–54.
Cardoso JO, Oliveira RV, Lu JBL, Desta Z. In vitro metabolism of montelukast by cytochrome P450s and UDP-glucuronosyltransferases. Drug Metab Dispos. 2015;43(12):1905–16.
Ma Y, Fu Y, Khojasteh SC, Dalvie D, Zhang D. Glucuronides as potential anionic substrates of human cytochrome P450 2C8 (CYP2C8). J Med Chem. 2017;60(21):8691–705.
Parkinson A, Kazmi F, Buckley DB, Yerino P, Ogilvie BW, Paris BL. System-dependent outcomes during the evaluation of drug candidates as inhibitors of cytochrome P450 (CYP) and uridine diphosphate glucuronosyltransferase (UGT) enzymes: human hepatocytes versus liver microsomes versus recombinant enzymes. Drug Metab Pharmacokinet. 2010;25(1):16–27.
Liu Y, Coughtrie MWH. Revisiting the latency of uridine diphosphate-glucuronosyltransferases (UGTs)-how does the endoplasmic reticulum membrane influence their function? Pharmaceutics. 2017;9(3):32.
Jaakkola T, Backman JT, Neuvonen M, Neuvonen PJ. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics of pioglitazone. Clin Pharmacol Ther. 2005;77(5):404–14.
Itkonen MK, Tornio A, Neuvonen M, Neuvonen PJ, Niemi M, Backman JT. Clopidogrel markedly increases plasma concentrations of CYP2C8 substrate pioglitazone. Drug Metab Dispos. 2016;44(8):1364–71.
Backman JT, Kyrklund C, Neuvonen M, Neuvonen PJ. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin Pharmacol Ther. 2002;72(6):685–91.
Banfield C, Hunt T, Reyderman L, Statkevich P, Padhi D, Affrime M. Lack of clinically relevant interaction between desloratadine and erythromycin. Clin Pharmacokinet. 2002;41Suppl 1:29–35.
Banfield C, Herron J, Keung A, Padhi D, Affrime M. Desloratadine has no clinically relevant electrocardiographic or pharmacodynamic interactions with ketoconazole. Clin Pharmacokinet. 2002;41Suppl 1:37–44.
Cheng H, Leff JA, Amin R, Gertz BJ, Smet MD, Noonan N, Rogers JD, Malbecq W, Meisner D, Somers G. Pharmacokinetics, bioavailability, and safety of montelukast sodium (MK-0476) in healthy males and females. Pharm Res. 1996;13(3):445–8.
Molimard M, Diquet B, Benedetti MS. Comparison of pharmacokinetics and metabolism of desloratadine, fexofenadine, levocetirizine and mizolastine in humans. Fundam Clin Pharmacol. 2004;18(4):399–411.
Hu G, Johnson EF, Kemper B. CYP2C8 exists as a dimer in natural membranes. Drug Metab Dispos. 2010;38(11):1976–83.
Fujiwara R, Yokoi T, Nakajima M. Structure and protein-protein interactions of human UDP-glucuronosyltransferases. Front Pharmacol. 2016;7:388.
Uchiyama M, Fischer T, Mueller J, Oguchi M, Yamamura N, Koda H, Iwabuchi H, Izumi T. Identification of novel metabolic pathways of pioglitazone in hepatocytes: N-glucuronidation of thiazolidinedione ring and sequential ring-opening pathway. Drug Metab Dispos. 2010;38(6):946–56.
Säll C, Houston JB, Galetin A. A comprehensive assessment of repaglinide metabolic pathways: impact of choice of in vitro system and relative enzyme contribution to in vitro clearance. Drug Metab Dispos. 2012;40(7):1279–89.
Schirris TJJ, Ritschel T, Bilos A, Smeitink JAM, Russel FGM. Statin lactonization by uridine 5’-diphospho-glucuronosyltransferases (UGTs). Mol Pharm. 2015;12(11):4048–55.
Varma MVS, Lai Y, Kimoto E, Goosen TC, El-Kattan AF, Kumar V. Mechanistic modeling to predict the transporter- and enzyme-mediated drug-drug interactions of repaglinide. Pharm Res. 2013;30(4):1188–99.
Kudo T, Hisaka A, Sugiyama Y, Ito K. Analysis of the repaglinide concentration increase produced by gemfibrozil and itraconazole based on the inhibition of the hepatic uptake transporter and metabolic enzymes. Drug Metab Dispos. 2013;41(2):362–71.
Kim SJ, Toshimoto K, Yao Y, Yoshikado T, Sugiyama Y. Quantitative analysis of complex drug-drug interactions between repaglinide and cyclosporin A/gemfibrozil using physiologically based pharmacokinetic models with in vitro transporter/enzyme inhibition data. J Pharm Sci. 2017;106(9):2715–26.
Yao Y, Toshimoto K, Kim SJ, Yoshikado T, Sugiyama Y. Quantitative analysis of complex drug-drug interactions between cerivastatin and metabolism/transport inhibitors using physiologically based pharmacokinetic modeling. Drug Metab Dispos. 2018;46(7):924–33.
Lee D, Doddapaneni S. Clinical pharmacology and biopharmaceutics reviews, FDA drug approval package of rozerem (ramelteon) tablets (application number 21-782). Available from URL: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021782s000_Rozerem_biopharmr.pdf. Accessed 27 Oct 2023.
Acknowledgements
We acknowledge the existence of a preprint version, available on Research Square (https://assets.researchsquare.com/files/rs-2364592/v1_coveres.pdf?c=1673300686), which shares a 39% overlap with this manuscript. The preprint has not undergone peer review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of Interest
KI and AK declare that they have no conflict of interest.
Data Availability
Additional data are given in the electronic supplementary material.
Author’s Contributions
KI contributed to the study conception and design. Data collection and analysis were performed by KI and AK. The first draft of the manuscript was written by KI, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Generative AI in Writing Process
During the preparation of this work, the authors used the generative AI (ChatGPT or Bing) in order to improve language and readability. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iga, K., Kiriyama, A. Interplay of UDP-Glucuronosyltransferase and CYP2C8 for CYP2C8 Mediated Drug Oxidation and Its Impact on Drug–Drug Interaction Produced by Standardized CYP2C8 Inhibitors, Clopidogrel and Gemfibrozil. Clin Pharmacokinet 63, 43–56 (2024). https://doi.org/10.1007/s40262-023-01322-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01322-7