Abstract
Aim
To apply model-informed drug development (MIDD) approach to support the decision making in drug development and accelerate the clinical development of janagliflozin, an orally selective SGLT2 inhibitor.
Method
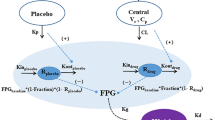
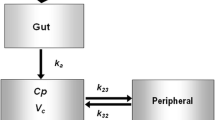
We previously developed a mechanistic pharmacokinetic/pharmacodynamic (PK/PD) model of janagliflozin based on preclinical data to optimize dose design in the first-in-human (FIH) study. In the current study, we used clinical PK/PD data of the FIH study to validate the model and then simulate the PK/PD profiles of multiple ascending dosing (MAD) study in healthy subjects. Besides, we developed a population PK/PD model of janagliflozin to predict steady-state urinary glucose excretion (UGE [UGE,ss]) in healthy subjects in the Phase 1 stage. This model was subsequently used to simulate the UGE, ss in patients with type 2 diabetes mellitus (T2DM) based on a unified PD target (ΔUGEc) across healthy subjects and patients with T2DM. This unified PD target was estimated from our previous work of model-based meta-analysis (MBMA) for the same class of drugs. The model-simulated UGE,ss in patients with T2DM was validated by data from the clinical Phase 1e study. Finally, at the end of the Phase 1 study, we simulated the 24-week hemoglobin A1c (HbA1c) level in patients with T2DM of janagliflozin based on the quantitative UGE/FPG/HbA1c relationship informed by our previous MBMA study for the same class of drugs.
Results
The pharmacologically active dose (PAD) levels of multiple ascending dosing (MAD) study were estimated to be 25, 50,100 mg once daily (QD) for 14 days based on the effective PD target of approximately 50 g daily UGE in healthy subjects. Besides, our previous MBMA analysis for the same class of drugs has provided a unified effective PD target of ΔUGEc approximately 0.5–0.6 g/(mg/dL) in both healthy subjects and patients with T2DM. In this study, the model-simulated steady-state ΔUGEc (ΔUGEc,ss) of janagliflozin in patients with T2DM were 0.52, 0.61 and 0.66 g/(mg/dL) for 25, 50, 100 mg QD dose levels. Finally, we estimated that HbA1c at 24 weeks would decrease 0.78 and 0.93 from baseline for the 25 and 50 mg QD dose groups.
Conclusions
The application of MIDD strategy adequately supported the decision making at each stage of janagliflozin development process. A waiver of Phase 2 study was successfully approved for janagliflozin based on these model-informed results and suggestions. This MIDD strategy of janagliflozin could be further utilized to support the clinical development of other SGLT2 inhibitors.





Similar content being viewed by others
References
Ogurtsova K, Guariguata L, Barengo NC, Ruiz PL, Sacre JW, Karuranga S, et al. IDF diabetes Atlas: global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract. 2022;183:109118. https://doi.org/10.1016/j.diabres.2021.109118.
Reed J, Bain S, Kanamarlapudi V. A review of current trends with type 2 diabetes epidemiology, aetiology, pathogenesis, treatments and future perspectives. Diabetes Metab Syndr Obes Targets Therapy. 2021;14:3567–602. https://doi.org/10.2147/dmso.S319895.
Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580–91. https://doi.org/10.1016/j.ophtha.2021.04.027.
Doyle-Delgado K, Chamberlain JJ, Shubrook JH, Skolnik N, Trujillo J. Pharmacologic approaches to glycemic treatment of type 2 diabetes: synopsis of the 2020 American Diabetes Association’s Standards of Medical Care in Diabetes Clinical Guideline. Ann Intern Med. 2020;173(10):813–21. https://doi.org/10.7326/m20-2470.
Marx N, Davies MJ, Grant PJ, Mathieu C, Petrie JR, Cosentino F, et al. Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol. 2021;9(1):46–52. https://doi.org/10.1016/s2213-8587(20)30343-0.
Khunti K, Jabbour S, Cos X, Mudaliar S, Mende C, Bonaca M, et al. Sodium-glucose cotransporter-2 (SGLT-2) inhibitors in patients with type 2 diabetes: barriers and solutions for improving uptake in routine clinical practice. Diabetes Obes Metab. 2022. https://doi.org/10.1111/dom.14684.
Berg DD, Docherty KF, Sattar N, Jarolim P, Welsh P, Jhund PS, et al. Serial assessment of high-sensitivity cardiac troponin and the effect of dapagliflozin in patients with heart failure with reduced ejection fraction: an analysis of the DAPA-HF Trial. Circulation. 2022;145(3):158–69. https://doi.org/10.1161/circulationaha.121.057852.
Light PE. Decoding the effects of SGLT2 inhibitors on cardiac arrhythmias in heart failure. Eur Heart J. 2021;42(36):3739–40. https://doi.org/10.1093/eurheartj/ehab563.
Curtain JP, Docherty KF, Jhund PS, Petrie MC, Inzucchi SE, Køber L, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J. 2021;42(36):3727–38. https://doi.org/10.1093/eurheartj/ehab560.
Song L, Yao X, Liu Y, Zhong W, Jiang J, Liu H, et al. Translational prediction of first-in-human pharmacokinetics and pharmacodynamics of janagliflozin, a selective SGLT2 inhibitor, using allometric scaling, dedrick and PK/PD modeling methods. Eur J Pharm Sci. 2020;147:105281. https://doi.org/10.1016/j.ejps.2020.105281.
Marshall SF, Burghaus R, Cosson V, Cheung SY, Chenel M, DellaPasqua O, et al. Good practices in model-informed drug discovery and development: practice, application, and documentation. CPT Pharmacomet Syst Pharmacol. 2016;5(3):93–122. https://doi.org/10.1002/psp4.12049.
Fang L, Kim MJ, Li Z, Wang Y, DiLiberti CE, Au J, et al. Model-informed drug development and review for generic products: summary of FDA Public Workshop. Clin Pharmacol Ther. 2018;104(1):27–30. https://doi.org/10.1002/cpt.1065.
Li X, Zhu X, Liu J, Li Q, Zhang H, Li C, et al. Pharmacokinetics, pharmacodynamics and tolerability of single and multiple doses of janagliflozin, a sodium-glucose co-transporter-2 inhibitor, in Chinese people with type 2 diabetes mellitus. Diabetes Obes Metab. 2020;22(12):2316–24. https://doi.org/10.1111/dom.14156.
Kaku K, Yamamoto K, Fukushima Y, Lliev H, Yasui A. Safety and effectiveness of empagliflozin in Japanese patients with type 2 diabetes: final results of a 3-year post-marketing surveillance study. Expert Opin Drug Saf. 2022. https://doi.org/10.1080/14740338.2022.2054987.
Kasichayanula S, Liu X, Lacreta F, Griffen SC, Boulton DW. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet. 2014;53(1):17–27. https://doi.org/10.1007/s40262-013-0104-3.
Komoroski B, Vachharajani N, Boulton D, Kornhauser D, Geraldes M, Li L, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther. 2009;85(5):520–6. https://doi.org/10.1038/clpt.2008.251.
Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85(5):513–9. https://doi.org/10.1038/clpt.2008.250.
Scheen AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Pharmacokinet. 2014;53(3):213–25. https://doi.org/10.1007/s40262-013-0126-x.
Shyangdan DS, Uthman OA, Waugh N. SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. BMJ Open. 2016;6(2):e009417. https://doi.org/10.1136/bmjopen-2015-009417.
Maurer TS, Ghosh A, Haddish-Berhane N, Sawant-Basak A, Boustany-Kari CM, She L, et al. Pharmacodynamic model of sodium-glucose transporter 2 (SGLT2) inhibition: implications for quantitative translational pharmacology. AAPS J. 2011;13(4):576–84. https://doi.org/10.1208/s12248-011-9297-2.
FDA. Clinical pharmacology and biopharmaceutics review of canagliflozin. Center for Drug Evaluation and Research. 2012;Reference ID: 3256450; https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204042Orig1s000ClinPharmR.pdf. Accessed 2 Feb 2023.
FDA. Clinical pharmacology and biopharmaceutics review of dapagliflozin. Center for Drug Evaluation and Research. 07/11/2013;Reference ID: 3423696; https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/202293orig1s000clinpharmr.pdf. Accessed 2 Feb 2023.
FDA. Clinical pharmacology and biopharmaceutics review of empagliflozin. Center for Drug Evaluation and Research. 07/18/2014;Reference ID: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/204629Orig1s000ClinPharmR.pdf. Accessed 2 Feb 2023.
Seino Y, Kaku K, Inagaki N, Haneda M, Sasaki T, Fukatsu A, et al. Fifty-two-week long-term clinical study of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Endocr J. 2015;62(7):593–603. https://doi.org/10.1507/endocrj.EJ15-0097.
Madabushi R, Seo P, Zhao L, Tegenge M, Zhu H. Review: role of model-informed drug development approaches in the lifecycle of drug development and regulatory decision-making. Pharm Res. 2022;39(8):1669–80. https://doi.org/10.1007/s11095-022-03288-w.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
The study was supported by the National Natural Science Foundation of China (No. 81903717), The Bill & Melinda Gates Foundation, Grant/Award Number: INV-007625, and The Peking University Third Hospital Clinical Project, Grant/Award Number: BYSYFY2021006.
Conflict of Interest
Jingfang Sun and Xinyu Hu are employees in Huisheng Bio-pharmaceutical (Jilin) Ltd., the company owing janagliflozin. Ling Song, Xiaoxu Wang, Haiyan Li, Pei Hu and Dongyang Liu, declare no conflicts of interest that might be relevant to the contents of this manuscript.
Ethical Approval
The data used in this study were collected in line with the principles of the Declaration of Helsinki. Approval was granted by institutional review boards and independent ethics committees for each study from which data were used in this work.
Data Availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Authors’ contributions
Dr. Ling Song, Dongyang Liu and Pei Hu designed the study, Dr. Ling Song, Xiaoxu Wang conducted the modeling and simulation work. Dr. Jingfang Sun and Xinyu Hu offered the preclinical and clinical raw data. Dr.Haiyan Li gave the writing suggestion for this article.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, L., Wang, X., Sun, J. et al. A Model-Informed Approach to Accelerate the Clinical Development of Janagliflozin, an Innovative SGLT2 Inhibitor. Clin Pharmacokinet 62, 505–518 (2023). https://doi.org/10.1007/s40262-022-01209-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01209-z