Abstract
Purpose
This study evaluated the effect of body mass index (BMI) on pharmacokinetic (PK) and pharmacodynamic (PD) parameters of insulin degludec in healthy Chinese males, depending on an euglycemic glucose clamp study.
Methods
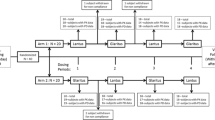
Sixty-five healthy male subjects were divided into four groups according to quartile of BMI value. Group A: BMI ≤ 20.7 kg/m2; group B: 20.7 < BMI ≤ 22.5 kg/m2; group C: 22.5 < BMI ≤ 23.6 kg/m2; group D: BMI > 23.6 kg/m2. Each volunteer received a single subcutaneous dose (0.4 U/kg) of insulin degludec and accepted a 24-h euglycemic glucose clamp study. The primary PK parameters were maximum observed drug concentration (Cmax) and the area under the curve (AUCINS) for the specified time intervals. The primary PD parameters were the time to the start of glucose infusion (Tonset), maximal glucose infusion rate (GIRmax) and area under the curve (AUCGIR) for the specified time intervals. The differences of these PK/PD parameters were compared among groups.
Results
Cmax and the AUC of insulin (0–6 h, 6–12 h and 0–24 h) were more than onefold higher in group A than those in groups B, C, D, and the concentration-time curve of group A was significantly shifted to the left compared with the other three groups. The GIRmax, total AUCGIR, and AUCGIR for each time interval were significantly higher in group A than those in other three groups. The proportion of AUCGIR in group A was the lowest proportion among four groups seen in the late stage. Multiple linear regression analysis showed that BMI was negatively correlated with AUCGIR,0-24 h.
Conclusions
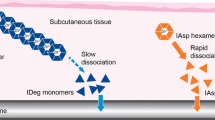
Insulin degludec in healthy Chinese male subjects with BMI ≤ 20.7 kg/m2 had a faster absorption, clearance, and a stronger glucose-lowering effect, but a steeper decrease of insulin action in the late stage after dosing.

Similar content being viewed by others
References
Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;28(369): m997.
Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29(8):2104–14.
Heise T, Hovelmann U, Nosek L, Hermanski L, Bottcher SG, Haahr H. Comparison of the pharmacokinetic and pharmacodynamic profiles of insulin degludec and insulin glargine. Expert Opin Drug Metab Toxicol. 2015;11(8):1193–201.
Zinman B, Philis-Tsimikas A, Cariou B, Handelsman Y, Rodbard HW, Johansen T, et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long). Diabetes Care. 2012;35(12):2464–71.
Jockel JP, Roebrock P, Shergold OA. Insulin depot formation in subcutaneoue tissue. J Diabetes Sci Technol. 2013;7(1):227–37.
Pitt JP, McCarthy OM, Hoeg-Jensen T, Wellman BM, Bracken RM. Factors influencing insulin absorption around exercise in type 1 diabetes. Front Endocrinol (Lausanne). 2020;11: 573275.
Gradel AKJ, Porsgaard T, Lykkesfeldt J, Seested T, Gram-Nielsen S, Kristensen NR, Refsgaard HHF. Factors affecting the absorption of subcutaneously administered insulin: effect on variability. J Diabetes Res. 2018;2018:1205121.
Kim GR, Choi D-W, Nam CM, Jang S-I, Park E-C. Synergistic association of high-sensitivity C-reactive protein and body mass index with insulin resistance in non-diabetic adults. Sci Rep. 2020;10:1.
Ashwell SG, Gebbie J, Home PD. Twice-daily compared with once-daily insulin glargine in people with Type 1 diabetes using meal-time insulin aspart. Diabet Med. 2006;23(8):879–86.
Heise T, Meiffren G, Alluis B, Seroussi C, Ranson A, Arrubla J, et al. BioChaperone Lispro versus faster aspart and insulin aspart in patients with type 1 diabetes using continuous subcutaneous insulin infusion: a randomized euglycemic clamp study. Diabetes Obes Metab. 2019;21(4):1066–70.
Hovelmann U, Raiter Y, Chullikana A, Liu M, Donnelly C, Lawrence T, et al. Pharmacokinetic and pharmacodynamic bioequivalence of biosimilar MYL-1601D with US and European insulin aspart in healthy volunteers: A randomized, double-blind, crossover, euglycaemic glucose clamp study. Diabetes Obes Metab. 2021;23(12):2670–8.
Widom B, Diamond MP, Simonson DC. Alterations in glucose metabolism during menstrual cycle in women with IDDM. Diabetes Care. 1992;15(2):213–20.
Gin H, Hanaire-Broutin H. Reproducibility and variability in the action of injected insulin. Diabetes Metab. 2005;31(1):7–13.
Vora JP, Burch A, Peters JR, Owens DR. Relationship between absorption of radiolabeled soluble insulin, subcutaneous blood flow, and anthropometry. Diabetes Care. 1992;15(11):1484–93.
Hildebrandt P. Skinfold thickness, local subcutaneous blood flow and insulin absorption in diabetic patients. Acta Physiol Scand Suppl. 1991;603:41–5.
Gobato AO, Vasques AC, Zambon MP, Barros Filho A, Hessel G. Metabolic syndrome and insulin resistance in obese adolescents. Rev Paul Pediatr. 2014;32(1):55–62.
Brody GH, Yu T, Chen E, Ehrlich KB, Miller GE. Racial discrimination, body mass index, and insulin resistance: a longitudinal analysis. Health Psychol. 2018;37(12):1107–14.
Ohnishi H, Saitoh S, Takagi S, Ohata J, Takeuchi H, Isobe T, et al. Incidence of insulin resistance in obese subjects in a rural Japanese population: the Tanno and Sobetsu study. Diabetes Obes Metab. 2005;7(1):83–7.
Lee MW, Fujioka K. Dietary prescriptions for the overweight patient: the potential benefits of low-carbohydrate diets in insulin resistance. Diabetes Obes Metab. 2011;13(3):204–6.
Haahr H, Heise T. A review of the pharmacological properties of insulin degludec and their clinical relevance. Clin Pharmacokinet. 2014;53(9):787–800.
Heller S, Buse J, Fisher M, Garg S, Marre M, Merker L, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1489–97.
Garber AJ, King AB, Prato SD, Sreenan S, Balci MK, Muñoz-Torres M, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1498–507.
Liu W, Yang X, Huang J. Efficacy and safety of insulin degludec versus insulin glargine: a systematic review and meta-analysis of fifteen clinical trials. Int J Endocrinol. 2018;2018:8726046.
Heise T, Korsatko S, Nosek L, Coester HV, Deller S, Roepstorff C, et al. Steady state is reached within 2–3 days of once-daily administration of degludec, a basal insulin with an ultralong duration of action. J Diabetes. 2016;8(1):132–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Ting Li, Hui Liu, Songlin Li, Hongling Yu, Jiaqi Li, Huiwen Tan, and Yerong Yu declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Author contributions
All the authors contributed to the study design, conduct, and/or data collection. TL and HL: study design, conduct, data collection, data analysis and writing manuscript. SL, HY, JL and HT: study design, conduct, data collection. YY: study design, supervision and revising manuscript.
Ethics approval
This study was performed in accordance with Good Clinical Practice and conformed to the ethical principles of the Declaration of Helsinki. Meanwhile the study was approved by an independent ethics committee of the West China Hospital of Sichuan University. All subjects wrote informed consent after a detailed explanation of the study protocol.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, T., Liu, H., Li, S. et al. The Effect of BMI on Pharmacokinetic and Pharmacodynamic Parameters of Insulin Degludec: Results from an Euglycemic Glucose Clamp Study. Clin Pharmacokinet 62, 449–456 (2023). https://doi.org/10.1007/s40262-022-01207-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01207-1