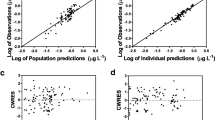
Abstract
Background
Levosimendan (LVSMD) is a calcium-sensitizer inotropic and vasodilator agent whose use might have a beneficial effect on the weaning of venoarterial extracorporeal membrane oxygenation (VA-ECMO). In light of LVSMD pharmacological characteristics, we hypothesized that ECMO may induce major pharmacokinetic (PK) modifications for LVSMD and its metabolites.
Objective
The aim of this study was to investigate the PK of LVSMD and its metabolites, and to assess the effects of ECMO on PK parameters.
Methods
We conducted a multicentric, prospective study (NCT03681379). Twenty-seven infusions of LVSMD were performed, allowing for the collection of 255 blood samples. Non-linear mixed-effects modeling software (MONOLIX®) was used to develop a parent-metabolite PK model of LVSMD and its metabolites.
Results
Most patients received a 0.2 µg/kg/min infusion of LVSMD over 24 h. After elimination of non-reliable samples or concentrations below the limit of quantification, 166, 101 and 85 samples were considered for LVSMD, OR-1855 and OR-1896, respectively, of which 81, 53 and 41, respectively, were drawn under ECMO conditions. Parent-metabolite PK modeling revealed that a two-compartment model with first-order elimination best described LVSMD PK. Use of a transit compartment allowed for an explanation of the delayed appearance of circulating OR-1855 and OR-1896, with the latter following a first-order elimination. Patient weight influenced the central volume of distribution and elimination of LVSMD. ECMO support increased the elimination rate of LVSMD by 78%, and ECMO also slowed down the metabolite formation rate by 85% for OR-1855, which in turn is converted to the active metabolite OR-1896, 14% slower than without ECMO. Simulated data revealed that standard dosing may not be appropriate for patients under ECMO, with a decrease in the steady-state concentration of LVSMD and lower exposure to the active metabolite OR-1896.
Conclusions
ECMO altered PK parameters for LVSMD and its metabolites. An infusion of LVSMD over 48 h, instead of 24 h, with a slightly higher dose may promote synthesis of the active metabolite OR-1896, which is responsible for the long-term efficacy of LVSMD. Further trials evaluating ECMO effects using a PK/pharmacodynamic approach may be of interest.
Registration: ClinicalTrials.gov identifier number NCT03681379.



Similar content being viewed by others
Change history
08 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40262-023-01218-6
References
Barbaro RP, Paden ML, Guner YS, Raman L, Ryerson LM, Alexander P, et al. Pediatric extracorporeal life support organization registry international report 2016. ASAIO J. 2017;63(4):456–63.
Barbaro RP, Boonstra PS, Paden ML, Roberts LA, Annich GM, Bartlett RH, et al. Development and validation of the pediatric risk estimate score for children using extracorporeal respiratory support (Ped-RESCUERS). Intensive Care Med. 2016;42(5):879–88.
Bembea MM, Felling R, Anton B, Salorio CF, Johnston MV. Neuromonitoring during extracorporeal membrane oxygenation: a systematic review of the literature. Pediatr Crit Care Med. 2015;16(6):558–64.
Dalton HJ, Reeder R, Garcia-Filion P, Holubkov R, Berg RA, Zuppa A, et al. Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2017;196(6):762–71.
Kaddoura R, Omar AS, Ibrahim MIM, Alkhulaifi A, Lorusso R, Elsherbini H, et al. The effectiveness of levosimendan on veno-arterial extracorporeal membrane oxygenation management and outcome: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2021;35(8):2483–95.
Silvetti S, Belletti A, Bianzina S, Momeni M. Effect of levosimendan treatment in pediatric patients with cardiac dysfunction: an update of a systematic review and meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. 2022;36(3):657–64.
Wang A, Cui C, Fan Y, Zi J, Zhang J, Wang G, et al. Prophylactic use of levosimendan in pediatric patients undergoing cardiac surgery: a prospective randomized controlled trial. Crit Care. 2019;23(1):428.
Figgitt DP, Gillies PS, Goa KL. Levosimendan. Drugs. 2001;61(5):613–27.
Wilson ID, Nicholson JK. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res. 2017;179:204–22.
Antila S, Sundberg S, Lehtonen LA. Clinical pharmacology of levosimendan. Clin Pharmacokinet. 2007;46(7):535–52.
Antila S, Kivikko M, Lehtonen L, Eha J, Heikkila A, Pohjanjousi P, et al. Pharmacokinetics of levosimendan and its circulating metabolites in patients with heart failure after an extended continuous infusion of levosimendan. Br J Clin Pharmacol. 2004;57(4):412–5.
Antila S, Pesonen U, Lehtonen L, Tapanainen P, Nikkanen H, Vaahtera K, et al. Pharmacokinetics of levosimendan and its active metabolite OR-1896 in rapid and slow acetylators. Eur J Pharm Sci. 2004;23(3):213–22.
Kaddoura R, Mohamed Ibrahim MI, Omar A. Levosimendan for VA-ECMO weaning: the silver lining. ESC Heart Fail. 2022;9(1):236–40.
Fang M, Cao H, Wang Z. Levosimendan in patients with cardiogenic shock complicating myocardial infarction: a meta-analysis. Med Intensiva (Engl Ed). 2018;42(7):409–15.
Wang H, Luo Q, Li Y, Zhang L, Wu X, Yan F. Effect of prophylactic levosimendan on all-cause mortality in pediatric patients undergoing cardiac surgery-an updated systematic review and meta-analysis. Front Pediatr. 2020;8:456.
Grześk G, Wołowiec Ł, Rogowicz D, Gilewski W, Kowalkowska M, Banach J, et al. The importance of pharmacokinetics, pharmacodynamic and repetitive use of levosimendan. Biomed Pharmacother. 2022;153: 113391.
Tukacs M. Pharmacokinetics and extracorporeal membrane oxygenation in adults: a literature review. AACN Adv Crit Care. 2018;29(3):246–58.
Thakkar N, Salerno S, Hornik CP, Gonzalez D. Clinical pharmacology studies in critically ill children. Pharm Res. 2017;34(1):7–24.
ICH guideline M10 on bioanalytical method validation and study sample analysis. Step 5 [cited 4 Nov 2022]. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf
Traynard P, Ayral G, Twarogowska M, Chauvin J. Efficient pharmacokinetic modeling workflow with the monolixsuite: a case study of remifentanil. CPT Pharmacometrics Syst Pharmacol. 2020;9(4):198–210.
Mould D, Upton R. Basic concepts in population modeling, simulation, and model-based drug development. Pharmacometr Syst Pharmacol. 2012;1(9):6.
Delattre M, Lavielle M, Poursat MA. A note on BIC in mixed-effects models. Electron J Stat. 2014;8(1):456–75.
Zhou H. Pharmacokinetic strategies in deciphering atypical drug absorption profiles. J Clin Pharmacol. 2003;43(3):211–27.
Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34(5):711–26.
Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37(3):840–51.
Pellicer A, Riera J, Lopez-Ortego P, Bravo MC, Madero R, Perez-Rodriguez J, et al. Phase 1 study of two inodilators in neonates undergoing cardiovascular surgery. Pediatr Res. 2013;73(1):95–103.
Wildschut ED, Ahsman MJ, Allegaert K, Mathot RAA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36(12):2109–16.
Shekar K, Roberts JA, Barnett AG, Diab S, Wallis SC, Fung YL, et al. Can physicochemical properties of antimicrobials be used to predict their pharmacokinetics during extracorporeal membrane oxygenation? Illustrative data from ovine models. Crit Care. 2015;19(1):437.
Shekar K, Roberts JA, Mcdonald CI, Ghassabian S, Anstey C, Wallis SC, et al. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study. Crit Care. 2015;19:164.
Raffaeli G, Pokorna P, Allegaert K, Mosca F, Cavallaro G, Wildschut ED, et al. Drug disposition and pharmacotherapy in neonatal ECMO: from fragmented data to integrated knowledge. Front Pediatr. 2019;7:360.
Papp Z, Csapó K, Pollesello P, Haikala H, Édes I. Pharmacological mechanisms contributing to the clinical efficacy of levosimendan. Cardiovasc Drug Rev. 2005;23(1):71–98.
Szilágyi S, Pollesello P, Levijoki J, Kaheinen P, Haikala H, Édes I, et al. The effects of levosimendan and OR-1896 on isolated hearts, myocyte-sized preparations and phosphodiesterase enzymes of the guinea pig. Eur J Pharmacol. 2004;486(1):67–74.
Puttonen J, Laine T, Ramela M, Häkkinen S, Zhang W, Pradhan R, et al. Pharmacokinetics and excretion balance of OR-1896, a pharmacologically active metabolite of levosimendan, in healthy men. Eur J Pharm Sci. 2007;32(4–5):271–7.
Chan CC, Lee KT, Ho WJ, Chan YH, Chu PH. Levosimendan use in patients with acute heart failure and reduced ejection fraction with or without severe renal dysfunction in critical cardiac care units: a multi-institution database study. Ann Intensive Care. 2021;11(1):27.
Tholén M, Ricksten SE, Lannemyr L. Effects of levosimendan on renal blood flow and glomerular filtration in patients with acute kidney injury after cardiac surgery: a double blind, randomized placebo-controlled study. Crit Care. 2021;25(1):207.
Cui X, Wang Z, Dong X, Cheng Z, Zhang L, Mu Y, et al. Comparative effectiveness and safety of milrinone and levosimendan as initial inotrope therapy in patients with acute heart failure with renal dysfunction. J Cardiovasc Pharmacol. 2022;79(6):781–90.
Puttonen J, Kantele S, Ruck A, Ramela M, Häkkinen S, Kivikko M, et al. Pharmacokinetics of intravenous levosimendan and its metabolites in subjects with hepatic impairment. J Clin Pharmacol. 2008;48(4):445–54.
Jonsson EN, Antila S, McFadyen L, Lehtonen L, Karlsson MO. Population pharmacokinetics of levosimendan in patients with congestive heart failure. Br J Clin Pharmacol. 2003;55(6):544–51.
Lunghetti S, Palmerini E, Urselli R, Maffei S, Guarino E, Focardi M, et al. Effects of levosimendan without loading dose on systolic and diastolic function in patients with end-stage heart failure. Cardiol J. 2011;18(5):532–7.
Kabbani TA, Pallav K, Dowd SE, Villafuerte-Galvez J, Vanga RR, Castillo NE, et al. Prospective randomized controlled study on the effects of Saccharomyces boulardii CNCM I-745 and amoxicillin-clavulanate or the combination on the gut microbiota of healthy volunteers. Gut Microbes. 2017;8(1):17–32.
Ellouze O, Soudry Faure A, Radhouani M, Abou-Arab O, Besnier E, Moussa M, et al. Levosimendan in venoarterial ECMO weaning. Rational and design of a randomized double blind multicentre trial. ESC Heart Fail. 2021;8(4):3339–47.
Assistance Publique - Hôpitaux de Paris. LEVOSIMENDAN to Facilitate Weaning From ECMO in Severe Cardiogenic Shock Patients. ClinicalTrials.gov; 2021 Oct. Report No.: NCT04728932 [cited 9 Sep 2022]. Available at: https://clinicaltrials.gov/ct2/show/NCT04728932
Vally S, Ferdynus C, Persichini R, Bouchet B, Braunberger E, Lo Pinto H, et al. Impact of levosimendan on weaning from peripheral venoarterial extracorporeal membrane oxygenation in intensive care unit. Ann Intensive Care. 2019;9(1):24.
Acknowledgements
The authors are grateful to the technical staff of the Laboratory of Pharmacokinetics of Rouen University Hospital for their support, and to all the physician’s staff who helped collect blood samples during their night-shifts. PB warmly thanks Club ECMO Pediatrique, Arcothova, and Dr. Ph. Mauriat for his help. Finally, the authors are grateful to Mr and Mrs Fox for their help in editing the present manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflicts of interest
Pierre Bourgoin’s institution received funding from Orion Pharma, Espoo, Finland. Jules Lecomte, Mehdi Oualha, Lionel Berthomieu, Tony Pereira, Emeline Davril, Fabien Lamoureux, Nicolas Joram, Alexis Chenouard, and Thomas Duflot declare they have no potential conflicts of interest.
Ethics approval
The study was approved by an independent review board (CPP IV Ile de France, reference 018-A02042-53). This study was conducted in accordance with the Helsinki Declaration.
Consent to participate
Parents provided written informed consent during the inclusion visit.
Consent for publication
Not applicable.
Code availability
Not applicable.
Data availability
All data generated or analyzed during this study are included in this published article and its online resources. Feel free to contact the corresponding author if needed.
Author contribution
PB and TD: Conceptualization, Data Curation, Analysis, Funding acquisition, Investigation, Writing, editing and Reviewing the manuscript. JL: Data curation, Analysis. MO, LB, NJ and AC: local investigators, Reviewing of the manuscript. TP, ED, FL: laboratory investigations.
Additional information
The original online version of this article was revised to correct the author name Mehdi Oualha.
Supplementary Information
The following information can be found in the Supplementary Information file available from the journal home page: S1, table: characteristics of patients supported with ECMO, S2, table: overview of BICc for various model tested,S3, figures a, b and c representing individual fits for LVSMD, OR-1855 and OR-1896 respectively, S4 and S5, csv file and word document: data available and list of abreviations, S6, figure: examples of clinical scenarios and corresponding modelizations (see text for details). Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bourgoin, P., Lecomte, J., Oualha, M. et al. Population Pharmacokinetics of Levosimendan and its Metabolites in Critically Ill Neonates and Children Supported or Not by Extracorporeal Membrane Oxygenation. Clin Pharmacokinet 62, 335–348 (2023). https://doi.org/10.1007/s40262-022-01199-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01199-y