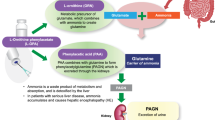
Abstract
Background and Objective
Givosiran, approved for the treatment of acute hepatic porphyria (AHP), is the first subcutaneously administered RNAi therapeutic. This analysis was undertaken to describe the plasma pharmacokinetics (PK) of givosiran and its active metabolite, AS(N-1)3′ givosiran, and to identify factors that contribute to intersubject PK variability.
Methods
A population PK model was developed using data from givosiran clinical trials that enrolled patients with AHP or who were asymptomatic chronic high excreters (CHEs) of toxic heme intermediates. Givosiran and AS(N-1)3′ givosiran PK were modeled simultaneously using non-linear mixed-effects modeling.
Results
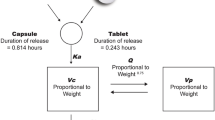
Plasma PK of givosiran was best described by a two-compartment model. Givosiran absorption after subcutaneous administration and conversion of givosiran to AS(N-1)3′ givosiran were incorporated as first-order processes. Hepatic clearance was the major route of elimination from the central compartment, with renal clearance accounting for < 20% of the total clearance. Body weight, East Asian ethnicity, and renal impairment were significant covariates in the model; however, none of the covariates evaluated resulted in clinically meaningful differences in plasma exposures of givosiran and AS(N-1)3′ givosiran. The model adequately described observed concentrations and variability across a wide range of dose levels. Model-derived simulations showed similar exposures for givosiran and its active metabolite in adults and adolescents.
Conclusions
The PK of givosiran and its active metabolite were not significantly affected by demographic or clinical parameters that would require adjustment from the approved body weight-based dose of givosiran 2.5 mg/kg once monthly.





Similar content being viewed by others
References
Phillips JD. Heme biosynthesis and the porphyrias. Mol Genet Metab. 2019;128(3):164–77.
de Souza PVS, Badia BML, Farias IB, Pinto W, Oliveira ASB. Acute hepatic porphyria: pathophysiological basis of neuromuscular manifestations. Front Neurosci. 2021;15: 715523.
Elder G, Harper P, Badminton M, Sandberg S, Deybach JC. The incidence of inherited porphyrias in Europe. J Inherit Metab Dis. 2013;36(5):849–57.
Anderson KE, Bloomer JR, Bonkovsky HL, Kushner JP, Pierach CA, Pimstone NR, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142(6):439–50.
Bissell DM, Wang B. Acute hepatic porphyria. J Clin Transl Hepatol. 2015;3(1):17–26.
GIVLAARI (givosiran) injection, for subcutaneous use [prescribing information]. Cambridge, MA: Alnylam Pharmaceuticals, Inc.; 2021.
GIVLAARI - Summary of Product Characteristics. Amsterdam: Alnylam Netherlands B.V.; 2021.
Balwani M, Sardh E, Ventura P, Peiro PA, Rees DC, Stolzel U, et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N Engl J Med. 2020;382(24):2289–301.
Ventura P, Bonkovsky HL, Gouya L, Aguilera-Peiro P, Montgomery Bissell D, Stein PE, et al. Efficacy and safety of givosiran for acute hepatic porphyria: 24-month interim analysis of the randomized phase 3 ENVISION study. Liver Int. 2022;42(1):161–72.
Sardh E, Harper P, Balwani M, Stein P, Rees D, Bissell DM, et al. Phase 1 trial of an RNA interference therapy for acute intermittent porphyria. N Engl J Med. 2019;380(6):549–58.
Agarwal S, Simon AR, Goel V, Habtemariam BA, Clausen VA, Kim JB, et al. Pharmacokinetics and pharmacodynamics of the small interfering ribonucleic acid, givosiran, in patients with acute hepatic porphyria. Clin Pharmacol Ther. 2020;108(1):63–72.
Li J, Liu J, Zhang X, Clausen V, Tran C, Arciprete M, et al. Nonclinical pharmacokinetics and absorption, distribution, metabolism, and excretion of givosiran, the first approved n-acetylgalactosamine-conjugated RNA interference therapeutic. Drug Metab Dispos. 2021;49(7):572–80.
Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136(49):16958–61.
McDougall R, Ramsden D, Agarwal S, Agarwal S, Aluri K, Arciprete M, et al. The nonclinical disposition and pharmacokinetic/pharmacodynamic properties of N-acetylgalactosamine-conjugated small interfering RNA are highly predictable and build confidence in translation to human. Drug Metab Dispos. 2022;50(6):781–97.
Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82(1):17–20.
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry: Pharmacokinetics in Patients with Impaired Renal Function—Study Design, Data Analysis, and Impact on Dosing. Spring Hill, MD: CDER; 2020.
Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481–504.
Vora JP, Burch A, Peters JR, Owens DR. Relationship between absorption of radiolabeled soluble insulin, subcutaneous blood flow, and anthropometry. Diabetes Care. 1992;15(11):1484–93.
Lazareth H, Poli A, Bignon Y, Mirmiran A, Rabant M, Cohen R, et al. Renal function decline with small interfering RNA silencing aminolevulinic acid synthase 1 (ALAS1). Kidney Int Rep. 2021;6(7):1904–11.
Ricci A, Guida CC, Manzini P, Cuoghi C, Ventura P. Kidney involvement in acute hepatic porphyrias: Pathophysiology and diagnostic implications. Diagnostics (Basel). 2021;11(12):2324.
Acknowledgements
Medical writing support, funded by Alnylam Pharmaceuticals, was provided by Crystal Murcia, PhD, of Inkwell Medical Communications LLC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was sponsored by Alnylam Pharmaceuticals.
Conflicts of interest
Megan Melch, Jongtae Lee, and Gabriel Robbie are Alnylam Pharmaceutical employees and may hold Alnylam stocks or options. Claudia Jomphe is an employee of Certara Strategic Consulting.
Ethics approval
All study protocols received Institutional Review Board or Ethics Committee approval and were conducted in accordance with the principles of the International Conference on Harmonisation Good Clinical Practice guidelines, the World Health Organization Declaration of Helsinki, and the 1996 Health Insurance Portability and Accountability Act.
Consent to participate
All study participants provided written informed consent.
Consent for publication
Not applicable.
Availability of data and material
De-identified individual participant data access will be provided contingent upon the approval of a research proposal and execution of a data sharing agreement. Requests for access to data can be submitted via the website http://www.vivli.org.
Code availability
Not applicable.
Author contributions
Gabriel J. Robbie was involved in the conception and design of the study. All authors were involved in the analysis and interpretation of the data, critical review of manuscript drafts, and approval of the submitted manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Melch, M., Lee, J., Jomphe, C. et al. Population Pharmacokinetic Analysis of the RNAi Therapeutic Givosiran in Patients with Acute Hepatic Porphyria. Clin Pharmacokinet 62, 89–99 (2023). https://doi.org/10.1007/s40262-022-01197-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01197-0