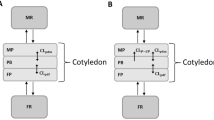
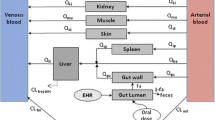
Abstract
Background
Concerns over maternal and fetal drug exposure during pregnancy highlight the need for improved understanding of drug distribution to the fetus through the placental barrier.
Objective
Our objective was to predict maternal and fetal drug disposition using a physiologically based pharmacokinetic (PBPK) modeling approach.
Methods
We used the detailed maternal–placental–fetal PBPK model within the Simcyp Simulator V20 to predict the maternal and fetal drug exposure of acyclovir, emtricitabine, lamivudine, and metformin during pregnancy and at delivery. The dynamic model includes gestational changes to the maternal, fetal, and placental physiological parameters. Placental kinetics were parameterized using published ex vivo data for these four compounds. Amniotic data were included where available. PBPK predictions were compared with the observed data using twofold criteria.
Results
Maternal–fetal PBPK models were developed completely from the bottom up without any parameter adjustments. The PBPK model-predicted exposures matched the observed maternal and umbilical exposure for acyclovir (six maternal studies, all of which all reported umbilical exposure), emtricitabine (six maternal studies, of which four reported umbilical exposure), lamivudine, (five maternal studies, of which four reported umbilical exposure), and metformin (seven studies, of which six reported umbilical exposure). Predicted pharmacokinetic parameters were within twofold of the observed values.
Conclusion
Integration of fetal and maternal system parameters within PBPK models, together with experimental data from ex vivo placental perfusion studies, facilitated and extended the application of the pregnancy PBPK model. Such models can also be used inform clinical trials and maternal/fetal risk assessment following maternally administered drugs or unintended exposure to environmental toxicants.








Similar content being viewed by others
References
Herring C, McManus A, Weeks A. Off-label prescribing during pregnancy in the UK: an analysis of 18,000 prescriptions in Liverpool Women’s Hospital. Int J Pharm Pract. 2010;18(4):226–9.
Laroche ML, Blin A, Coubret A, Grau M, Roux B, Aubard Y. Off-label prescribing during pregnancy in France: the NeHaVi cohort. Int J Clin Pharmacol Ther. 2020;58(4):198–208.
Rayburn WF, Turnbull GL. Off-label drug prescribing on a state university obstetric service. J Reprod Med. 1995;40(3):186–8.
Bouazza N, Foissac F, Hirt D, Urien S, Benaboud S, Lui G, et al. Methodological approaches to evaluate fetal drug exposure. Curr Pharm Des. 2019;25(5):496–504.
Schmidt A, Morales-Prieto DM, Pastuschek J, Frohlich K, Markert UR. Only humans have human placentas: molecular differences between mice and humans. J Reprod Immunol. 2015;108:65–71.
De Sousa MM, Lui G, Zheng Y, Pressiat C, Hirt D, Valade E, et al. A physiologically-based pharmacokinetic model to predict human fetal exposure for a drug metabolized by several CYP450 pathways. Clin Pharmacokinet. 2017;56(5):537–50.
Schalkwijk S, Buaben AO, Freriksen JJM, Colbers AP, Burger DM, Greupink R, et al. Prediction of fetal darunavir exposure by integrating human ex-vivo placental transfer and physiologically based pharmacokinetic modeling. Clin Pharmacokinet. 2018;57(6):705–16.
Abduljalil K, Badhan RKS. Drug dosing during pregnancy-opportunities for physiologically based pharmacokinetic models. J Pharmacokinet Pharmacodyn. 2020;47(4):319–40.
Abduljalil K, Furness P, Johnson TN, Rostami-Hodjegan A, Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: a database for parameters required in physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2012;51(6):365–96.
Abduljalil K, Johnson TN, Rostami-Hodjegan A. Fetal physiologically-based pharmacokinetic models: systems information on fetal biometry and gross composition. Clin Pharmacokinet. 2018;57(9):1149–71.
Abduljalil K, Jamei M, Johnson TN. Fetal physiologically based pharmacokinetic models: systems information on the growth and composition of fetal organs. Clin Pharmacokinet. 2019;58(2):235–62.
Abduljalil K, Pan X, Clayton R, Johnson TN, Jamei M. Fetal physiologically based pharmacokinetic models: systems information on fetal cardiac output and its distribution to different organs during development. Clin Pharmacokinet. 2021;60(6):741–57.
Abduljalil K, Jamei M, Johnson TN. Fetal physiologically based pharmacokinetic models: systems information on fetal blood components and binding proteins. Clin Pharmacokinet. 2020;59(5):629–42.
Zhang Z, Imperial MZ, Patilea-Vrana GI, Wedagedera J, Gaohua L, Unadkat JD. Development of a novel maternal-fetal physiologically based pharmacokinetic model I: insights into factors that determine fetal drug exposure through simulations and sensitivity analyses. Drug Metab Dispos. 2017;45(8):920–38.
Abduljalil K, Pan X, Pansari A, Jamei M, Johnson TN. A preterm physiologically based pharmacokinetic model part I: physiological parameters and model building. Clin Pharmacokinet. 2020;59(4):485–500.
Barker G, Boyd RD, D’Souza SW, Donnai P, Fox H, Sibley CP. Placental water content and distribution. Placenta. 1994;15(1):47–56.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57.
Neuhoff S, Gaohua L, Burt H, Jamei M, Li L, Tucker GT, et al. Accounting for transporters in renal clearance: towards a mechanistic kidney model (Mech KiM). In: Sugiyama Y, Steffansen B, editors. Transporters in drug development AAPS advances in the pharmaceutical sciences series. Springer, New York. 2013.
Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24(1):67–76.
Blackburn S. Maternal, fetal and neonatal physiology: a clinical perspective. 3rd ed. Philadelphia: Saunders Elsevier; 2007.
Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. 2005;25(5):341–8.
Abduljalil K, Pansari A, Jamei M. Prediction of maternal pharmacokinetics using physiologically based pharmacokinetic models: assessing the impact of the longitudinal changes in the activity of CYP1A2, CYP2D6 and CYP3A4 enzymes during pregnancy. J Pharmacokinet Pharmacodyn. 2020;47(4):361–83.
Haddad J, Langer B, Astruc D, Messer J, Lokiec F. Oral acyclovir and recurrent genital herpes during late pregnancy. Obstet Gynecol. 1993;82(1):102–4.
Leung DT, Henning PA, Wagner EC, Blasig A, Wald A, Sacks SL, et al. Inadequacy of plasma acyclovir levels at delivery in patients with genital herpes receiving oral acyclovir suppressive therapy in late pregnancy. J Obstet Gynaecol Can. 2009;31(12):1137–43.
Kimberlin DF, Weller S, Whitley RJ, Andrews WW, Hauth JC, Lakeman F, et al. Pharmacokinetics of oral valacyclovir and acyclovir in late pregnancy. Am J Obstet Gynecol. 1998;179(4):846–51.
Frenkel LM, Brown ZA, Bryson YJ, Corey L, Unadkat JD, Hensleigh PA, et al. Pharmacokinetics of acyclovir in the term human pregnancy and neonate. Am J Obstet Gynecol. 1991;164(2):569–76.
Matsuzaki T, Scotcher D, Darwich AS, Galetin A, Rostami-Hodjegan A. Towards further verification of physiologically-based kidney models: predictability of the effects of urine-flow and urine-ph on renal clearance. J Pharmacol Exp Ther. 2019;368(2):157–68.
Laskin OL, Longstreth JA, Saral R, de Miranda P, Keeney R, Lietman PS. Pharmacokinetics and tolerance of acyclovir, a new anti-herpesvirus agent, in humans. Antimicrob Agents Chemother. 1982;21(3):393–8.
Vergin H, Kikuta C, Mascher H, Metz R. Pharmacokinetics and bioavailability of different formulations of aciclovir. Arzneimittelforschung. 1995;45(4):508–15.
Liao MZ, Flood Nichols SK, Ahmed M, Clark S, Hankins GD, Caritis S, et al. Effects of Pregnancy on the Pharmacokinetics of Metformin. Drug Metab Dispos. 2020;48(4):264–71.
Eyal S, Easterling TR, Carr D, Umans JG, Miodovnik M, Hankins GD, et al. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos. 2010;38(5):833–40.
Bergagnini-Kolev MC, Hebert MF, Easterling TR, Lin YS. Pregnancy Increases the renal secretion of N(1)-methylnicotinamide, an endogenous probe for renal cation transporters, in patients prescribed metformin. Drug Metab Dispos. 2017;45(3):325–9.
Henderson GI, Hu ZQ, Johnson RF, Perez AB, Yang Y, Schenker S. Acyclovir transport by the human placenta. J Lab Clin Med. 1992;120(6):885–92.
Gilstrap LC, Bawdon RE, Roberts SW, Sobhi S. The transfer of the nucleoside analog ganciclovir across the perfused human placenta. Am J Obstet Gynecol. 1994;170(4):967–72.
Soul-Lawton J, Seaber E, On N, Wootton R, Rolan P, Posner J. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother. 1995;39(12):2759–64.
Lewis LD, Fowle AS, Bittiner SB, Bye A, Isaacs PE. Human gastrointestinal absorption of acyclovir from tablet duodenal infusion and sipped solution. Br J Clin Pharmacol. 1986;21(4):459–62.
Amini H, Javan M, Gazerani P, Ghaffari A, Ahmadiani A. Lack of bioequivalence between two aciclovir tablets in healthy subjects. Clin Drug Investig. 2008;28(1):47–53.
Gilead Sciences. Emtriva® prescribing information. 2012. http://www.gilead.com/pdf/emtriva_pi.pdf. Accessed 5 Apr 2021.
Hirt D, Urien S, Rey E, Arrive E, Ekouevi DK, Coffie P, et al. Population pharmacokinetics of emtricitabine in human immunodeficiency virus type 1-infected pregnant women and their neonates. Antimicrob Agents Chemother. 2009;53(3):1067–73.
De Sousa MM, Chetty M. Are standard doses of renally-excreted antiretrovirals in older patients appropriate: a PBPK study comparing exposures in the elderly population with those in renal impairment. Drugs R D. 2019;19(4):339–50.
De Sousa MM, Hirt D, Vinot C, Valade E, Lui G, Pressiat C, et al. Prediction of human fetal pharmacokinetics using ex vivo human placenta perfusion studies and physiologically based models. Br J Clin Pharmacol. 2016;81(4):646–57.
Zong J, Chittick GE, Wang LH, Hui J, Begley JA, Blum MR. Pharmacokinetic evaluation of emtricitabine in combination with other nucleoside antivirals in healthy volunteers. J Clin Pharmacol. 2007;47(7):877–89.
Blum MR, Chittick GE, Begley JA, Zong J. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J Clin Pharmacol. 2007;47(6):751–9.
Wang LH, Begley J, St Claire RL, Harris J, Wakeford C, Rousseau FS. Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection. AIDS Res Hum Retroviruses. 2004;20(11):1173–82.
Rousseau FS, Kahn JO, Thompson M, Mildvan D, Shepp D, Sommadossi JP, et al. Prototype trial design for rapid dose selection of antiretroviral drugs: an example using emtricitabine (Coviracil). J Antimicrob Chemother. 2001;48(4):507–13.
Valade E, Treluyer JM, Bouazza N, Ghosn J, Foissac F, Benaboud S, et al. Population pharmacokinetics of emtricitabine in HIV-1-infected adult patients. Antimicrob Agents Chemother. 2014;58(4):2256–61.
Stek AM, Best BM, Luo W, Capparelli E, Burchett S, Hu C, et al. Effect of pregnancy on emtricitabine pharmacokinetics. HIV Med. 2012;13(4):226–35.
Colbers AP, Hawkins DA, Gingelmaier A, Kabeya K, Rockstroh JK, Wyen C, et al. The pharmacokinetics, safety and efficacy of tenofovir and emtricitabine in HIV-1-infected pregnant women. AIDS. 2013;27(5):739–48.
Liu XI, Momper JD, Rakhmanina N, van den Anker JN, Green DJ, Burckart GJ, et al. Physiologically based pharmacokinetic models to predict maternal pharmacokinetics and fetal exposure to emtricitabine and acyclovir. J Clin Pharmacol. 2020;60(2):240–55.
Valade E, Treluyer JM, Dabis F, Arrive E, Pannier E, Benaboud S, et al. Modified renal function in pregnancy: impact on emtricitabine pharmacokinetics. Br J Clin Pharmacol. 2014;78(6):1378–86.
Johnson MA, Moore KH, Yuen GJ, Bye A, Pakes GE. Clinical pharmacokinetics of lamivudine. Clin Pharmacokinet. 1999;36(1):41–66.
FDA. Lamuvidine ID: 4157751. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020564s37_020596s036lbl.pdf.
Benaboud S, Treluyer JM, Urien S, Blanche S, Bouazza N, Chappuy H, et al. Pregnancy-related effects on lamivudine pharmacokinetics in a population study with 228 women. Antimicrob Agents Chemother. 2012;56(2):776–82.
Chappuy H, Treluyer JM, Jullien V, Dimet J, Rey E, Fouche M, et al. Maternal-fetal transfer and amniotic fluid accumulation of nucleoside analogue reverse transcriptase inhibitors in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother. 2004;48(11):4332–6.
Mandelbrot L, Peytavin G, Firtion G, Farinotti R. Maternal-fetal transfer and amniotic fluid accumulation of lamivudine in human immunodeficiency virus-infected pregnant women. Am J Obstet Gynecol. 2001;184(2):153–8.
Moodley J, Moodley D, Pillay K, Coovadia H, Saba J, van Leeuwen R, et al. Pharmacokinetics and antiretroviral activity of lamivudine alone or when coadministered with zidovudine in human immunodeficiency virus type 1-infected pregnant women and their offspring. J Infect Dis. 1998;178(5):1327–33.
Yeh RF, Rezk NL, Kashuba AD, Dumond JB, Tappouni HL, Tien HC, et al. Genital tract, cord blood, and amniotic fluid exposures of seven antiretroviral drugs during and after pregnancy in human immunodeficiency virus type 1-infected women. Antimicrob Agents Chemother. 2009;53(6):2367–74.
Bloom SL, Dias KM, Bawdon RE, Gilstrap LC 3rd. The maternal-fetal transfer of lamivudine in the ex vivo human placenta. Am J Obstet Gynecol. 1997;176(2):291–3.
Challier JC. Criteria for evaluating perfusion experiments and presentation of results. Contrib Gynecol Obstet. 1985;13:32–9.
Yuen GJ, Morris DM, Mydlow PK, Haidar S, Hall ST, Hussey EK. Pharmacokinetics, absolute bioavailability, and absorption characteristics of lamivudine. J Clin Pharmacol. 1995;35(12):1174–80.
van Leeuwen R, Lange JM, Hussey EK, Donn KH, Hall ST, Harker AJ, et al. The safety and pharmacokinetics of a reverse transcriptase inhibitor, 3TC, in patients with HIV infection: a phase I study. AIDS. 1992;6(12):1471–5.
Heald AE, Hsyu PH, Yuen GJ, Robinson P, Mydlow P, Bartlett JA. Pharmacokinetics of lamivudine in human immunodeficiency virus-infected patients with renal dysfunction. Antimicrob Agents Chemother. 1996;40(6):1514–9.
Yuen GJ, Lou Y, Bumgarner NF, Bishop JP, Smith GA, Otto VR, et al. Equivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 milligrams once daily and 150 milligrams twice daily. Antimicrob Agents Chemother. 2004;48(1):176–82.
Charles B, Norris R, Xiao X, Hague W. Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit. 2006;28(1):67–72.
Hughes RC, Gardiner SJ, Begg EJ, Zhang M. Effect of pregnancy on the pharmacokinetics of metformin. Diabet Med. 2006;23(3):323–6.
Tertti K, Laine K, Ekblad U, Rinne V, Ronnemaa T. The degree of fetal metformin exposure does not influence fetal outcome in gestational diabetes mellitus. Acta Diabetol. 2014;51(5):731–8.
Kovo M, Haroutiunian S, Feldman N, Hoffman A, Glezerman M. Determination of metformin transfer across the human placenta using a dually perfused ex vivo placental cotyledon model. Eur J Obstet Gynecol Reprod Biol. 2008;136(1):29–33.
de Oliveira Baraldi C, Lanchote VL, de Jesus Antunes N, de Jesus Ponte Carvalho TM, Dantas Moises EC, Duarte G, et al. Metformin pharmacokinetics in nondiabetic pregnant women with polycystic ovary syndrome. Eur J Clin Pharmacol. 2011;67(10):1027–33.
Christensen T, Klebe JG, Bertelsen V, Hansen HE. Changes in renal volume during normal pregnancy. Acta Obstet Gynecol Scand. 1989;68(6):541–3.
Bailey RR, Rolleston GL. Kidney length and ureteric dilatation in the puerperium. J Obstet Gynaecol Br Commonw. 1971;78(1):55–61.
Reznicek J, Ceckova M, Cerveny L, Müller F, Staud F. Emtricitabine is a substrate of MATE1 but not of OCT1, OCT2, P-gp, BCRP or MRP2 transporters. Xenobiotica. 2017;47(1):77–85.
Bousquet L, Pruvost A, Didier N, Farinotti R, Mabondzo A. Emtricitabine: Inhibitor and substrate of multidrug resistance associated protein. Eur J Pharm Sci. 2008;35(4):247–56.
De Sousa MM, Hirt D, Urien S, Valade E, Bouazza N, Foissac F, et al. Physiologically-based pharmacokinetic modeling of renally excreted antiretroviral drugs in pregnant women. Br J Clin Pharmacol. 2015;80(5):1031–41.
Ceckova M, Reznicek J, Ptackova Z, Cerveny L, Muller F, Kacerovsky M, et al. Role of ABC and solute carrier transporters in the placental transport of lamivudine. Antimicrob Agents Chemother. 2016;60(9):5563–72.
Choi MK, Jin QR, Jin HE, Shim CK, Cho DY, Shin JG, et al. Effects of tetraalkylammonium compounds with different affinities for organic cation transporters on the pharmacokinetics of metformin. Biopharm Drug Dispos. 2007;28(9):501–10.
Sata R, Ohtani H, Tsujimoto M, Murakami H, Koyabu N, Nakamura T, et al. Functional analysis of organic cation transporter 3 expressed in human placenta. J Pharmacol Exp Ther. 2005;315(2):888–95.
Anoshchenko O, Prasad B, Neradugomma NK, Wang J, Mao Q, Unadkat JD. Gestational age-dependent abundance of human placental transporters as determined by quantitative targeted proteomics. Drug Metab Dispos. 2020;48(9):735–41.
Kovo M, Kogman N, Ovadia O, Nakash I, Golan A, Hoffman A. Carrier-mediated transport of metformin across the human placenta determined by using the ex vivo perfusion of the placental cotyledon model. Prenat Diagn. 2008;28(6):544–8.
Tertti K, Ekblad U, Heikkinen T, Rahi M, Ronnemaa T, Laine K. The role of organic cation transporters (OCTs) in the transfer of metformin in the dually perfused human placenta. Eur J Pharm Sci. 2010;39(1–3):76–81.
Muller F, Weitz D, Mertsch K, Konig J, Fromm MF. Importance of OCT2 and MATE1 for the cimetidine-metformin interaction: insights from investigations of polarized transport in single- and double-transfected MDCK cells with a focus on perpetrator disposition. Mol Pharm. 2018;15(8):3425–33.
Dallmann A, Ince I, Solodenko J, Meyer M, Willmann S, Eissing T, et al. Physiologically based pharmacokinetic modeling of renally cleared drugs in pregnant women. Clin Pharmacokinet. 2017;56(12):1525–41.
Jogiraju VK, Avvari S, Gollen R, Taft DR. Application of physiologically based pharmacokinetic modeling to predict drug disposition in pregnant populations. Biopharm Drug Dispos. 2017;38(7):426–38.
Xia B, Heimbach T, Gollen R, Nanavati C, He H. A simplified PBPK modeling approach for prediction of pharmacokinetics of four primarily renally excreted and CYP3A metabolized compounds during pregnancy. AAPS J. 2013;15(4):1012–24.
Song L, Yu Z, Xu Y, Li X, Liu X, Liu D, et al. Preliminary physiologically based pharmacokinetic modeling of renally cleared drugs in Chinese pregnant women. Biopharm Drug Dispos. 2020;41(6):248–267. https://doi.org/10.1002/bdd.2243.
Mian P, Allegaert K, Conings S, Annaert P, Tibboel D, Pfister M, et al. Integration of placental transfer in a fetal-maternal physiologically based pharmacokinetic model to characterize acetaminophen exposure and metabolic clearance in the fetus. Clin Pharmacokinet. 2020;59(7):911–25.
Freriksen JJM, Schalkwijk S, Colbers AP, Abduljalil K, Russel FGM, Burger DM, et al. Assessment of maternal and fetal dolutegravir exposure by integrating ex vivo placental perfusion data and physiologically-based pharmacokinetic modeling. Clin Pharmacol Ther. 2020;107(6):1352–61.
Modena AB, Fieni S. Amniotic fluid dynamics. Acta Biomed. 2004;75(Suppl 1):11–3.
Hague WM, Davoren PM, McIntyre D, Norris R, Xiaonian XCB. Metformin crosses the placenta: a modulator for fetal insulin resistance? BMJ. 2003;327:880.
Acknowledgements
The authors thank Eleanor Savill and Anna Kenworthy for their assistance with collecting the references and preparing the manuscript and thank Ruth Clayton for helpful comments and proofreading.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this study.
Conflicts of interest
Khaled Abduljalil, Amita Pansari, Jia Ning, and Masoud Jamei are full-time employees of Certara UK Limited, Simcyp Division. The activities of Certara are supported by a consortium of pharmaceutical companies. The Simcyp Simulator is available at no cost, following completion of the training workshop, to approved members of academic institutions and other not-for-profit organizations for research and teaching purposes.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Availability material
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
KA was responsible for the conception and design of the study. KA, JN, and AP performed the experiments and acquired the data. KA, AP, JN, and MJ analyzed and interpreted the data. KA drafted or revised the manuscript critically for important intellectual content with input from all authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abduljalil, K., Pansari, A., Ning, J. et al. Prediction of Maternal and Fetal Acyclovir, Emtricitabine, Lamivudine, and Metformin Concentrations during Pregnancy Using a Physiologically Based Pharmacokinetic Modeling Approach. Clin Pharmacokinet 61, 725–748 (2022). https://doi.org/10.1007/s40262-021-01103-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-021-01103-0