Abstract
Background
Lower respiratory tract infections are common in adult patients with cystic fibrosis (CF) and are frequently caused by Pseudomonas aeruginosa, resulting in chronic lung inflammation and fibrosis. The progression of multidrug-resistant strains of P. aeruginosa and alterations in the pharmacokinetics of many antibiotics in CF make optimal antimicrobial therapy a challenge, as reflected by high between- and inter-individual variability (IIV).
Objectives
This review provides a synthesis of population pharmacokinetic models for various antibiotics prescribed in adult CF patients, and aims at identifying the most reported structural models, covariates and sources of variability influencing the dose–concentration relationship.
Methods
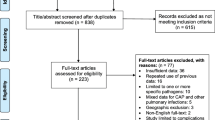
A literature search was conducted using the PubMed database, from inception to August 2020, and articles were retained if they met the inclusion/exclusion criteria.
Results
A total of 19 articles were included in this review. One-, two- and three-compartment models were reported to best describe the pharmacokinetics of various antibiotics. The most common covariates were lean body mass and creatinine clearance. After covariate inclusion, the IIV (range) in total body clearance was 27.2% (10.40–59.7%) and 25.9% (18.0–33.9%) for β-lactams and aminoglycosides, respectively. IIV in total body clearance was estimated at 36.3% for linezolid and 22.4% for telavancin. The IIV (range) in volume of distribution was 29.4% (8.8–45.9%) and 15.2 (11.6–18.0%) for β-lactams and aminoglycosides, respectively, and 26.9% for telavancin. The median (range) of residual variability for all studies, using a combined (proportional and additive) model, was 12.7% (0.384–30.80%) and 0.126 mg/L (0.007–1.88 mg/L), respectively.
Conclusion
This is the first review that highlights key aspects of different population pharmacokinetic models of antibiotics prescribed in adult CF patients, effectively proposing relevant information for clinicians and researchers to optimize antibiotic therapy in CF.
Similar content being viewed by others
References
Elborn JS. Cystic fibrosis. Lancet. 2016;388(10059):2519–31. https://doi.org/10.1016/s0140-6736(16)00576-6.
Lubamba B, Dhooghe B, Noel S, Leal T. Cystic fibrosis: insight into CFTR pathophysiology and pharmacotherapy. Clin Biochem. 2012;45(15):1132–44. https://doi.org/10.1016/j.clinbiochem.2012.05.034.
Cystic Fibrosis Canada. About CF: What is Cystic Fibrosis. http://www.fibrosekystique.ca/about-cf.
Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Child. 1938;56(2):344–99. https://doi.org/10.1001/archpedi.1938.01980140114013.
Cystic Fibrosis Foundation. Patient Registry Annual Data Report. Cystic Fibrosis Foundation; 2018.
Cystic Fibrosis Canada. The Canadian Cystic Fibrosis Registry 2018 Annual Data Report. Toronto: Cystic Fibrosis Foundation; 2018.
Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(8):918–51. https://doi.org/10.1164/rccm.200304-505SO.
Davies JC, Alton EW, Bush A. Cystic fibrosis. BMJ. 2007;335(7632):1255–9. https://doi.org/10.1136/bmj.39391.713229.AD.
Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245(4922):1073–80. https://doi.org/10.1126/science.2570460.
Pierre-Audigier C, Ferroni A, Sermet-Gaudelus I, Le Bourgeois M, Offredo C, Vu-Thien H, et al. Age-related prevalence and distribution of nontuberculous mycobacterial species among patients with cystic fibrosis. J Clin Microbiol. 2005;43(7):3467–70. https://doi.org/10.1128/jcm.43.7.3467-3470.2005.
Smith MJ, Efthimiou J, Hodson ME, Batten JC. Mycobacterial isolations in young adults with cystic fibrosis. Thorax. 1984;39(5):369–75. https://doi.org/10.1136/thx.39.5.369.
Eikani MS, Nugent M, Poursina A, Simpson P, Levy H. Clinical course and significance of nontuberculous mycobacteria and its subtypes in cystic fibrosis. BMC Infect Dis. 2018;18(1):311. https://doi.org/10.1186/s12879-018-3200-z.
Flume PA, Mogayzel PJ Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, et al. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180(9):802–8. https://doi.org/10.1164/rccm.200812-1845PP.
Waters V, Stanojevic S, Atenafu EG, Lu A, Yau Y, Tullis E, et al. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J. 2012;40(1):61–6. https://doi.org/10.1183/09031936.00159111.
de Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, Freitag A, et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax. 2011;66(8):680–5. https://doi.org/10.1136/thx.2011.161117.
Stephenson AL, Tom M, Berthiaume Y, Singer LG, Aaron SD, Whitmore GA, et al. A contemporary survival analysis of individuals with cystic fibrosis: a cohort study. Eur Respir J. 2015;45(3):670–9. https://doi.org/10.1183/09031936.00119714.
Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108(5):832–9. https://doi.org/10.1016/j.jada.2008.02.020.
VanDevanter DR, Konstan MW. Outcome measures for clinical trials assessing treatment of cystic fibrosis lung disease. Clin Investig (Lond). 2012;2(2):163–75. https://doi.org/10.4155/cli.11.174.
Emerson J, McNamara S, Buccat AM, Worrell K, Burns JL. Changes in cystic fibrosis sputum microbiology in the United States between 1995 and 2008. Pediatr Pulmonol. 2010;45(4):363–70. https://doi.org/10.1002/ppul.21198.
Touw DJ. Clinical pharmacokinetics of antimicrobial drugs in cystic fibrosis. Pharm World Sci. 1998;20(4):149–60. https://doi.org/10.1023/a:1008634911114.
Rey E, Treluyer JM, Pons G. Drug disposition in cystic fibrosis. Clin Pharmacokinet. 1998;35(4):313–29. https://doi.org/10.2165/00003088-199835040-00004.
de Groot R, Smith AL. Antibiotic pharmacokinetics in cystic fibrosis. Differences and clinical significance. Clin Pharmacokinet. 1987;13(4):228–53. https://doi.org/10.2165/00003088-198713040-00002.
Bulitta JB, Jiao Y, Drescher SK, Oliver A, Louie A, Moya B, et al. Four decades of beta-lactam antibiotic pharmacokinetics in cystic fibrosis. Clin Pharmacokinet. 2019;58(2):143–56. https://doi.org/10.1007/s40262-018-0678-x.
Thompson RZ, Martin CA, Burgess DR, Rutter WC, Burgess DS. Optimizing beta-lactam pharmacodynamics against Pseudomonas aeruginosa in adult cystic fibrosis patients. J Cyst Fibros. 2016;15(5):660–3. https://doi.org/10.1016/j.jcf.2016.04.002.
Young DC, Zobell JT, Stockmann C, Waters CD, Ampofo K, Sherwin CM, et al. Optimization of anti-pseudomonal antibiotics for cystic fibrosis pulmonary exacerbations: V. Aminoglycosides. Pediatr Pulmonol. 2013;48(11):1047–61. https://doi.org/10.1002/ppul.22813.
Zobell JT, Young DC, Waters CD, Ampofo K, Stockmann C, Sherwin CM, et al. Optimization of anti-pseudomonal antibiotics for cystic fibrosis pulmonary exacerbations: VI. Executive summary. Pediatr Pulmonol. 2013;48(6):525–37. https://doi.org/10.1002/ppul.22757.
Bauer LA. The aminoglycoside antibiotics. In: Bauer LA, editor. Applied clinical pharmacokinetics. 3rd ed. New York: McGraw-Hill Medical; 2015.
Marsot A, Guilhaumou R, Riff C, Blin O. Amikacin in critically ill patients: a review of population pharmacokinetic studies. Clin Pharmacokinet. 2017;56(2):127–38. https://doi.org/10.1007/s40262-016-0428-x.
Spino M. Pharmacokinetics of drugs in cystic fibrosis. Clin Rev Allergy. 1991;9(1–2):169–210. https://doi.org/10.1007/978-1-4612-0475-6_11.
Vinks AA, van Rossem RN, Mathot RA, Heijerman HG, Mouton JW. Pharmacokinetics of aztreonam in healthy subjects and patients with cystic fibrosis and evaluation of dose-exposure relationships using Monte Carlo simulation. Antimicrob Agents Chemother. 2007;51(9):3049–55. https://doi.org/10.1128/aac.01522-06.
Huls CE, Prince RA, Seilheimer DK, Bosso JA. Pharmacokinetics of cefepime in cystic fibrosis patients. Antimicrob Agents Chemother. 1993;37(7):1414–6. https://doi.org/10.1128/aac.37.7.1414.
de Velde F, Mouton JW, de Winter BCM, van Gelder T, Koch BCP. Clinical applications of population pharmacokinetic models of antibiotics: challenges and perspectives. Pharmacol Res. 2018;134:280–8. https://doi.org/10.1016/j.phrs.2018.07.005.
Jusko WJ, Mosovich LL, Gerbracht LM, Mattar ME, Yaffe SJ. Enhanced renal excretion of dicloxacillin in patients with cystic fibrosis. Pediatrics. 1975;56(6):1038–44.
Spino M, Chai RP, Isles AF, Thiessen JJ, Tesoro A, Gold R, et al. Cloxacillin absorption and disposition in cystic fibrosis. J Pediatr. 1984;105(5):829–35. https://doi.org/10.1016/s0022-3476(84)80317-0.
Leeder JS, Spino M, Isles AF, Tesoro AM, Gold R, MacLeod SM. Ceftazidime disposition in acute and stable cystic fibrosis. Clin Pharmacol Ther. 1984;36(3):355–62. https://doi.org/10.1038/clpt.1984.187.
de Groot R, Hack BD, Weber A, Chaffin D, Ramsey B, Smith AL. Pharmacokinetics of ticarcillin in patients with cystic fibrosis: a controlled prospective study. Clin Pharmacol Ther. 1990;47(1):73–8. https://doi.org/10.1038/clpt.1990.11.
Hedman A, Alvan G, Strandvik B, Arvidsson A. Increased renal clearance of cefsulodin due to higher glomerular filtration rate in cystic fibrosis. Clin Pharmacokinet. 1990;18(2):168–75. https://doi.org/10.2165/00003088-199018020-00006.
Yaffe SJ, Gerbracht LM, Mosovich LL, Mattar ME, Danish M, Jusko WJ. Pharmacokinetics of methicillin in patients with cystic fibrosis. J Infect Dis. 1977;135(5):828–31. https://doi.org/10.1093/infdis/135.5.828.
Seng Yue C, Ozdin D, Selber-Hnatiw S, Ducharme MP. Opportunities and challenges related to the implementation of model-based bioequivalence criteria. Clin Pharmacol Ther. 2019;105(2):350–62. https://doi.org/10.1002/cpt.1270.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Barsky EE, Pereira LM, Sullivan KJ, Wong A, McAdam AJ, Sawicki GS, et al. Ceftaroline pharmacokinetics and pharmacodynamics in patients with cystic fibrosis. J Cyst Fibros. 2018;17(3):e25–31. https://doi.org/10.1016/j.jcf.2017.10.010.
Han EE, Beringer PM, Falck P, Louie S, Rao P, Shapiro B, et al. Pilot study of continuous infusion cefepime in adult patients with cystic fibrosis. J Antimicrob Chemother. 2006;57(5):1017–9. https://doi.org/10.1093/jac/dkl053.
Kuti JL, Nightingale CH, Knauft RF, Nicolau DP. Pharmacokinetic properties and stability of continuous-infusion meropenem in adults with cystic fibrosis. Clin Ther. 2004;26(4):493–501. https://doi.org/10.1016/S0149-2918(04)90051-3.
Bulitta JB, Landersdorfer CB, Hüttner SJ, Drusano GL, Kinzig M, Holzgrabe U, et al. Population pharmacokinetic comparison and pharmacodynamic breakpoints of ceftazidime in cystic fibrosis patients and healthy volunteers. Antimicrob Agents Chemother. 2010;54(3):1275–82. https://doi.org/10.1128/aac.00936-09.
Bensman TJ, Wang J, Jayne J, Fukushima L, Rao AP, D’Argenio DZ, et al. Pharmacokinetic–pharmacodynamic target attainment analyses To determine optimal dosing of ceftazidime–avibactam for the treatment of acute pulmonary exacerbations in patients with cystic fibrosis. Antimicrob Agents Chemother. 2017;61(10):e00988-e1017. https://doi.org/10.1128/aac.00988-17.
Vinks AA, Mouton JW, Touw DJ, Heijerman HG, Danhof M, Bakker W. Population pharmacokinetics of ceftazidime in cystic fibrosis patients analyzed by using a nonparametric algorithm and optimal sampling strategy. Antimicrob Agents Chemother. 1996;40(5):1091–7. https://doi.org/10.1128/aac.40.5.1091.
Mouton JW, Punt N, Vinks AA. A retrospective analysis using Monte Carlo simulation to evaluate recommended ceftazidime dosing regimens in healthy volunteers, patients with cystic fibrosis, and patients in the intensive care unit. Clin Ther. 2005;27(6):762–72. https://doi.org/10.1016/j.clinthera.2005.06.013.
Monogue ML, Pettit RS, Muhlebach M, Cies JJ, Nicolau DP, Kuti JL. Population pharmacokinetics and safety of ceftolozane–tazobactam in adult cystic fibrosis patients admitted with acute pulmonary exacerbation. Antimicrob Agents Chemother. 2016;60(11):6578–84. https://doi.org/10.1128/aac.01566-16.
Kidd JM, Sakon CM, Oleksiuk LM, Cies JJ, Pettit RS, Nicolau DP, et al. Pharmacokinetics of telavancin in adult patients with cystic fibrosis during acute pulmonary exacerbation. Antimicrob Agents Chemother. 2019;64(1):e01914-e1919. https://doi.org/10.1128/aac.01914-19.
Bulitta JB, Duffull SB, Kinzig-Schippers M, Holzgrabe U, Stephan U, Drusano GL, et al. Systematic comparison of the population pharmacokinetics and pharmacodynamics of piperacillin in cystic fibrosis patients and healthy volunteers. Antimicrob Agents Chemother. 2007;51(7):2497–507. https://doi.org/10.1128/aac.01477-06.
Vinks AA, Den Hollander JG, Overbeek SE, Jelliffe RW, Mouton JW. Population pharmacokinetic analysis of nonlinear behavior of piperacillin during intermittent or continuous infusion in patients with cystic fibrosis. Antimicrob Agents Chemother. 2003;47(2):541–7. https://doi.org/10.1128/aac.47.2.541-547.2003.
Bulitta JB, Duffull SB, Landersdorfer CB, Kinzig M, Holzgrabe U, Stephan U, et al. Comparison of the pharmacokinetics and pharmacodynamic profile of carumonam in cystic fibrosis patients and healthy volunteers. Diagn Microbiol Infect Dis. 2009;65(2):130–41. https://doi.org/10.1016/j.diagmicrobio.2009.06.018.
Shah NR, Bulitta JB, Kinzig M, Landersdorfer CB, Jiao Y, Sutaria DS, et al. Novel population pharmacokinetic approach to explain the differences between cystic fibrosis patients and healthy volunteers via protein binding. Pharmaceutics. 2019;11(6):286. https://doi.org/10.3390/pharmaceutics11060286.
Bulitta JB, Kinzig M, Landersdorfer CB, Holzgrabe U, Stephan U, Sörgel F. Comparable population pharmacokinetics and pharmacodynamic breakpoints of cefpirome in cystic fibrosis patients and healthy volunteers. Antimicrob Agents Chemother. 2011;55(6):2927–36. https://doi.org/10.1128/AAC.01484-10.
Illamola SM, Huynh HQ, Liu X, Bhakta ZN, Sherwin CM, Liou TG, et al. Population pharmacokinetics of amikacin in adult patients with cystic fibrosis. Antimicrob Agents Chemother. 2018;62(10):e00877-e918. https://doi.org/10.1128/aac.00877-18.
Thirion DJG, Pasche V, Matouk E, Marsot A. Amikacin nomogram for treatment of adult cystic fibrosis exacerbations based on an external evaluation of a population pharmacokinetic model. Pediatr Pulmonol. 2020;55(5):1154–60. https://doi.org/10.1002/ppul.24689.
Touw DJ, Knox AJ, Smyth A. Population pharmacokinetics of tobramycin administered thrice daily and once daily in children and adults with cystic fibrosis. J Cyst Fibros. 2007;6(5):327–33. https://doi.org/10.1016/j.jcf.2006.12.007.
Hennig S, Standing JF, Staatz CE, Thomson AH. Population pharmacokinetics of tobramycin in patients with and without cystic fibrosis. Clin Pharmacokinet. 2013;52(4):289–301. https://doi.org/10.1007/s40262-013-0036-y.
Alghanem S, Paterson I, Touw DJ, Thomson AH. Influence of multiple courses of therapy on aminoglycoside clearance in adult patients with cystic fibrosis. J Antimicrob Chemother. 2013;68(6):1338–47. https://doi.org/10.1093/jac/dkt035.
Marsot A, Hraiech S, Cassir N, Daviet F, Parzy G, Blin O, et al. Aminoglycosides in critically ill patients: which dosing regimens for which pathogens? Int J Antimicrob Agents. 2020;56(4):106124. https://doi.org/10.1016/j.ijantimicag.2020.106124.
Touw DJ, Vinks AA, Neef C. Pharmacokinetic modelling of intravenous tobramycin in adolescent and adult patients with cystic fibrosis using the nonparametric expectation maximization (NPEM) algorithm. Pharm World Sci. 1997;19(3):142–51. https://doi.org/10.1023/a:1008633526772.
Keel RA, Schaeftlein A, Kloft C, Pope JS, Knauft RF, Muhlebach M, et al. Pharmacokinetics of intravenous and oral linezolid in adults with cystic fibrosis. Antimicrob Agents Chemother. 2011;55(7):3393–8. https://doi.org/10.1128/aac.01797-10.
Ting L, Aksenov S, Bhansali SG, Ramakrishna R, Tang P, Geller DE. Population pharmacokinetics of inhaled tobramycin powder in cystic fibrosis patients. CPT Pharmacometr Syst Pharmacol. 2014;3(2):e99. https://doi.org/10.1038/psp.2013.76.
Levison ME, Levison JH. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect Dis Clin N Am. 2009;23(4):791–815. https://doi.org/10.1016/j.idc.2009.06.008.
Antibiotics Cystic Fibrosis Foundation. Antibiotics. https://www.cff.org/Life-With-CF/Treatments-and-Therapies/Medications/Antibiotics/.
Castellani C, Duff AJA, Bell SC, Heijerman HGM, Munck A, Ratjen F, et al. ECFS best practice guidelines: the 2018 revision. J Cyst Fibros. 2018;17(2):153–78. https://doi.org/10.1016/j.jcf.2018.02.006.
Döring G, Flume P, Heijerman H, Elborn JS. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11(6):461–79. https://doi.org/10.1016/j.jcf.2012.10.004.
UCF Trust. Antibiotic treatment for cystic fibrosis. UK Cystic Fibrosis Trust Antibiotic Working Group; 2009.
Josephson SBGMB. BMJ Best Practice: Cystic Fibrosis. BMJ. 2020.
Central Drugs Standard Control Organisation. Drugs at CDSCO. https://cdscoonline.gov.in/CDSCO/Drugs.
Kakizaki H, Ishii N, Murakami S, Suzuki K, Takamizawa A, Hirano J, et al. Clinical evaluation of the combination of carumonam and fosfomycin in the treatment of complicated urinary tract infection [in Japanese]. Hinyokika Kiyo. 1990;36(6):731–5.
Vibativ® [package insert] South San Francisco, CA: Theravance Biopharma US, Inc; 2017.
Bernstein AT, Leigh MW, Goralski JL, Esther CR, McKinzie CJ. Use of telavancin in adolescent patients with cystic fibrosis and prior intolerance to vancomycin: a case series. J Cyst Fibros. 2018;17(6):e48–50. https://doi.org/10.1016/j.jcf.2018.08.003.
Adult Guide to Cystic Fibrosis. Cystic Fibrosis Foundation.
Marshall BC. Survival trending upward but what does this really mean? Cystic Fibrosis Foundation; 2017.
Harness-Brumley CL, Elliott AC, Rosenbluth DB, Raghavan D, Jain R. Gender differences in outcomes of patients with cystic fibrosis. J Womens Health (Larchmt). 2014;23(12):1012–20. https://doi.org/10.1089/jwh.2014.4985.
Goss CH, Rubenfeld GD, Ramsey BW, Aitken ML. Clinical trial participants compared with nonparticipants in cystic fibrosis. Am J Respir Crit Care Med. 2006;173(1):98–104. https://doi.org/10.1164/rccm.200502-273OC.
Yong J, Frost F, Nazareth D, Walshaw M. Case report: haemolytic anaemia with ceftazidime use in a patient with cystic fibrosis. F1000Res. 2018;7:475. https://doi.org/10.12688/f1000research.14505.1.
MacDougall C. Penicillins, cephalosporins, and other β-lactam antibiotics. In: Brunton LL, Hilal-Dandan R, Knollmann BC, editors. Goodman and Gilman’s: the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill Education; 2017.
Dimelow R, Wright JG, MacPherson M, Newell P, Das S. Population pharmacokinetic modelling of ceftazidime and avibactam in the plasma and epithelial lining fluid of healthy volunteers. Drugs R D. 2018;18(3):221–30. https://doi.org/10.1007/s40268-018-0241-0.
Healthcare Access and Quality Index. Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. Lancet. 2018;391(10136):2236–71. https://doi.org/10.1016/s0140-6736(18)30994-2.
Tikkanen RA, Melinda K. U.S. Health care from a global perspective, 2019: higher spending, worse outcomes? The Commonwealth Fund; 2020. https://www.commonwealthfund.org/publications/issue-briefs/2020/jan/us-health-care-global-perspective-2019.
Landersdorfer CB, Bulitta JB, Kirkpatrick CM, Kinzig M, Holzgrabe U, Drusano GL, et al. Population pharmacokinetics of piperacillin at two dose levels: influence of nonlinear pharmacokinetics on the pharmacodynamic profile. Antimicrob Agents Chemother. 2012;56(11):5715–23. https://doi.org/10.1128/aac.00937-12.
Fang CT, Chen YC, Lin SF, Shau WY, Liu CJ, Sheng WH, et al. Safety and efficacy of cefpirome in comparison with ceftazidime in chinese patients with sepsis due to bacterial infections. Chemotherapy. 2000;46(5):371–8. https://doi.org/10.1159/000007311.
Burckhardt G, Koepsell H. CHAPTER 73—Organic anion and cation transporters in renal elimination of drugs. In: Alpern RJ, Hebert SC, editors. Seldin and Giebisch’s the kidney. 4th ed. San Diego: Academic Press; 2008. p. 2045–80.
Beauduy CE, Winston LG. Beta-lactam and other cell wall- and membrane-active antibiotics. In: Katzung BG, Vanderah TW (eds). Basic clinical pharmacology, 15th edition. New York, NY: McGraw-Hill; 2021.
Prescott WA. A survey of extended-interval aminoglycoside dosing practices in united states adult cystic fibrosis programs. Respir Care. 2014;59(9):1353. https://doi.org/10.4187/respcare.02980.
Garraffo R, Drugeon HB, Dellamonica P, Bernard E, Lapalus P. Determination of optimal dosage regimen for amikacin in healthy volunteers by study of pharmacokinetics and bactericidal activity. Antimicrob Agents Chemother. 1990;34(4):614–21. https://doi.org/10.1128/aac.34.4.614.
Shah S, Barton G, Fischer A. Pharmacokinetic considerations and dosing strategies of antibiotics in the critically ill patient. J Intensive Care Soc. 2015;16(2):147–53. https://doi.org/10.1177/1751143714564816.
Soraluce A, Barrasa H, Asín-Prieto E, Sánchez-Izquierdo J, Maynar J, Isla A, et al. Novel population pharmacokinetic model for linezolid in critically ill patients and evaluation of the adequacy of the current dosing recommendation. Pharmaceutics. 2020;12(1):54. https://doi.org/10.3390/pharmaceutics12010054.
Morgan DJ, Bray KM. Lean body mass as a predictor of drug dosage. Clin Pharmacokinet. 1994;26(4):292–307. https://doi.org/10.2165/00003088-199426040-00005.
Touw DJ, Vinks AA, Heijerman HG, Hermans J, Bakker W. Suggestions for the optimization of the initial tobramycin dose in adolescent and adult patients with cystic fibrosis. Ther Drug Monit. 1994;16(2):125–31. https://doi.org/10.1097/00007691-199404000-00003.
Touw DJ, Vinks AA, Mouton JW, Horrevorts AM. Pharmacokinetic optimisation of antibacterial treatment in patients with cystic fibrosis. Current practice and suggestions for future directions. Clin Pharmacokinet. 1998;35(6):437–59. https://doi.org/10.2165/00003088-199835060-00003.
Guilhaumou R, Benaboud S, Bennis Y, Dahyot-Fizelier C, Dailly E, Gandia P, et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients—guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation—SFAR). Crit Care. 2019;23(1):104. https://doi.org/10.1186/s13054-019-2378-9.
Stalker DJ, Jungbluth GL. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin Pharmacokinet. 2003;42(13):1129–40. https://doi.org/10.2165/00003088-200342130-00004.
Gonzalez LS 3rd, Spencer JP. Aminoglycosides: a practical review. Am Fam Physician. 1998;58(8):1811–20.
Touw DJ, Vinks AA, Jacobs F, Heijerman HG, Bakker W. Creatinine clearance as predictor of tobramycin elimination in adult patients with cystic fibrosis. Ther Drug Monit. 1996;18(5):562–9. https://doi.org/10.1097/00007691-199610000-00007.
Jusko WJ. Perspectives on variability in pharmacokinetics of an oral contraceptive product. Contraception. 2017;95(1):5–9. https://doi.org/10.1016/j.contraception.2016.07.019.
Karlsson MO, Beal SL, Sheiner LB. Three new residual error models for population PK/PD analyses. J Pharmacokinet Biopharm. 1995;23(6):651–72. https://doi.org/10.1007/BF02353466.
Karvaly GB, Neely MN, Kovács K, Vincze I, Vásárhelyi B, Jelliffe RW. Development of a methodology to make individual estimates of the precision of liquid chromatography-tandem mass spectrometry drug assay results for use in population pharmacokinetic modeling and the optimization of dosage regimens. PLoS ONE. 2020;15(3):e0229873. https://doi.org/10.1371/journal.pone.0229873.
Graves DA, Locke CS, Muir KT, Miller RP. The influence of assay variability on pharmacokinetic parameter estimation. J Pharmacokinet Biopharm. 1989;17(5):571–92. https://doi.org/10.1007/BF01071350.
Karlsson MO, Sheiner LB. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm. 1993;21(6):735–50. https://doi.org/10.1007/bf01113502.
Savant AP, McColley SA. Cystic fibrosis year in review 2018, part 1. Pediatr Pulmonol. 2019;54(8):1117–28. https://doi.org/10.1002/ppul.24361.
Hong LT, Liou TG, Deka R, King JB, Stevens V, Young DC. Pharmacokinetics of continuous infusion beta-lactams in the treatment of acute pulmonary exacerbations in adult patients with cystic fibrosis. Chest. 2018;154(5):1108–14. https://doi.org/10.1016/j.chest.2018.06.002.
Information P. Tobramycin IV, IM injection, tobramycin IV, IM injection. Schaumburg: APP Pharmaceuticals, LLC; 2008.
Albright JC, Houck AP, Pettit RS. Effects of CFTR modulators on pharmacokinetics of tobramycin during acute pulmonary exacerbations in the pediatric cystic fibrosis population. Pediatr Pulmonol. 2020. https://doi.org/10.1002/ppul.24917 ((Epub 22 Jun 2020)).
US FDA. Guidance for Industry: Population Pharmacokinetics. 1999. http://www.fda.gov/cder/guidance/1852.fnl.pdf.
Ralph LD, Sandstrom M, Twelves C, Dobbs NA, Thomson AH. Assessment of the validity of a population pharmacokinetic model for epirubicin. Br J Clin Pharmacol. 2006;62(1):47–55. https://doi.org/10.1111/j.1365-2125.2006.02584.x.
Sun H, Fadiran EO, Jones CD, Lesko L, Huang S-M, Higgins K, et al. Population pharmacokinetics. Clin Pharmacokinet. 1999;37(1):41–58. https://doi.org/10.2165/00003088-199937010-00003.
Döring G, Conway SP, Heijerman HG, Hodson ME, Høiby N, Smyth A, et al. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur Respir J. 2000;16(4):749–67. https://doi.org/10.1034/j.1399-3003.2000.16d30.x.
Stokem K, Zuckerman JB, Nicolau DP, Wungwattana M, Sears EH. Use of ceftolozane–tazobactam in a cystic fibrosis patient with multidrug-resistant pseudomonas infection and renal insufficiency. Respir Med Case Rep. 2018;23:8–9. https://doi.org/10.1016/j.rmcr.2017.10.012.
Merk & Co Inc.. Zerbaxa (ceftolozane/tazobactam) [package insert]. US FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/206829s009lbl.pdf.
Mentré F, Escolano S. Prediction discrepancies for the evaluation of nonlinear mixed-effects models. J Pharmacokinet Pharmacodyn. 2006;33(3):345–67. https://doi.org/10.1007/s10928-005-0016-4.
Noone P, Parsons TM, Pattison JR, Slack RC, Garfield-Davies D, Hughes K. Experience in monitoring gentamicin therapy during treatment of serious Gram-negative sepsis. Br Med J. 1974;1(5906):477–81. https://doi.org/10.1136/bmj.1.5906.477.
Noone P, Pattison JR, Davies DG. The effective use of gentamicin in life-threatening sepsis. Postgrad Med J. 1974;50(Suppl 7):9–16.
Noone P, Rogers BT. Pneumonia caused by coliforms and Pseudomonas aeruginosa. J Clin Pathol. 1976;29(7):652–6. https://doi.org/10.1136/jcp.29.7.652.
Moore RD, Smith CR, Lietman PS. The association of aminoglycoside plasma levels with mortality in patients with Gram-negative bacteremia. J Infect Dis. 1984;149(3):443–8. https://doi.org/10.1093/infdis/149.3.443.
Moore RD, Smith CR, Lietman PS. Association of aminoglycoside plasma levels with therapeutic outcome in Gram-negative pneumonia. Am J Med. 1984;77(4):657–62. https://doi.org/10.1016/0002-9343(84)90358-9.
Kashuba AD, Nafziger AN, Drusano GL, Bertino JS Jr. Optimizing aminoglycoside therapy for nosocomial pneumonia caused by Gram-negative bacteria. Antimicrob Agents Chemother. 1999;43(3):623–9. https://doi.org/10.1128/aac.43.3.623.
Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155(1):93–9. https://doi.org/10.1093/infdis/155.1.93.
Forrest A, Nix DE, Ballow CH, Goss TF, Birmingham MC, Schentag JJ. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37(5):1073–81. https://doi.org/10.1128/aac.37.5.1073.
Forrest A, Chodosh S, Amantea MA, Collins DA, Schentag JJ. Pharmacokinetics and pharmacodynamics of oral grepafloxacin in patients with acute bacterial exacerbations of chronic bronchitis. J Antimicrob Chemother. 1997;40(Suppl A):45–57. https://doi.org/10.1093/jac/40.suppl_1.45.
Preston SL, Drusano GL, Berman AL, Fowler CL, Chow AT, Dornseif B, et al. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA. 1998;279(2):125–9. https://doi.org/10.1001/jama.279.2.125.
MacGowan A, White L, Reeves D, Harding I. Retrospective review of serum teicoplanin concentrations in clinical trials and their relationship to clinical outcome. J Infect Chemother. 1996;2(4):197–208. https://doi.org/10.1007/BF02355116.
Guillemot D, Carbon C, Balkau B, Geslin P, Lecoeur H, Vauzelle-Kervroëdan F, et al. Low dosage and long treatment duration of beta-lactam: risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA. 1998;279(5):365–70. https://doi.org/10.1001/jama.279.5.365.
MacGowan AP. Role of pharmacokinetics and pharmacodynamics: does the dose matter? Clin Infect Dis. 2001;33(Suppl 3):S238–9. https://doi.org/10.1086/321855.
Zobell JT, Waters CD, Young DC, Stockmann C, Ampofo K, Sherwin CM, et al. Optimization of anti-pseudomonal antibiotics for cystic fibrosis pulmonary exacerbations: II. Cephalosporins and penicillins. Pediatr Pulmonol. 2013;48(2):107–22. https://doi.org/10.1002/ppul.22669.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Mehdi El Hassani received a scholarship from Université de Montréal, and Amélie Marsot acknowledges support from the Fonds de Recherche du Québec-Santé (FRQS) Research Scholars—Junior 1 (Young Researcher Establishment) Career Scholarship.
Conflict of interest
Mehdi El Hassani, Jean-Alexandre Caissy, and Amélie Marsot declare no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
El Hassani, M., Caissy, JA. & Marsot, A. Antibiotics in Adult Cystic Fibrosis Patients: A Review of Population Pharmacokinetic Analyses. Clin Pharmacokinet 60, 447–470 (2021). https://doi.org/10.1007/s40262-020-00970-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-020-00970-3