Abstract
Background and Objective
Elagolix is an oral, non-peptide, gonadotropin-releasing hormone receptor antagonist. It is approved for the treatment of moderate-to-severe pain associated with endometriosis and is being investigated for the treatment of heavy menstrual bleeding associated with uterine fibroids. Use of low-dose hormonal add-back therapy can reduce hypoestrogenic effects associated with elagolix, thus there is a need to determine if there is a pharmacokinetic interaction between elagolix and low-dose hormonal add-back therapy.
Methods
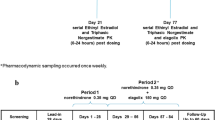
Two multiple-dose, open-label, single-sequence, non-randomized studies for elagolix 300 mg twice daily with oral (n = 24) and transdermal (n = 36) low-dose add-back therapy (estradiol [E2]/norethindrone acetate [NETA]; 1 mg/0.5 mg oral and 0.51 mg/4.8 mg transdermal) in healthy postmenopausal women were conducted, with pharmacokinetic sampling for E2, estrone (E1), and NETA up to 72 or 96 h after dosing. Pharmacokinetic parameters for hormones were estimated using noncompartmental methods.
Results
No change in norethindrone maximum plasma concentration or area under the concentration–time curve was observed when oral E2/NETA was administered with elagolix. For E2, there was a 2-fold increase in maximum plasma concentration and a 1.5-fold increase in the area under the concentration–time curve, and for E1 there was a 1.7-fold increase in maximum plasma concentration when oral E2/NETA was administered with elagolix. Exposures for norethindrone, E2, and E1 were unchanged when transdermal E2/NETA was applied with elagolix administration.
Conclusions
Although changes in E2/E1 exposures were observed when oral E2/NETA was co-administered with elagolix, these changes are not considered clinically relevant; and no dose adjustments are recommended when elagolix is co-administered with oral or transdermal low-dose add-back therapy.



Similar content being viewed by others

Availability of Data and Material
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g., protocols and clinical study reports), provided the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan and execution of a Data Sharing Agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
References
Bulun SE. Uterine fibroids. N Engl J Med. 2013;369(14):1344–55. https://doi.org/10.1056/NEJMra1209993.
Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017;124(10):1501–12. https://doi.org/10.1111/1471-0528.14640.
De La Cruz MS, Buchanan EM. Uterine fibroids: diagnosis and treatment. Am Fam Physician. 2017;95(2):100–7.
Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35(6):473–80. https://doi.org/10.1055/s-0037-1607264.
Soliman AM, Anand SB, Coyne KS, Castelli-Haley J, Snabes M, Owens CD. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59(10):974–81. https://doi.org/10.1097/JOM.0000000000001105.
Fuldeore MJ, Soliman AM. Patient-reported prevalence and symptomatic burden of uterine fibroids among women in the United States: findings from a cross-sectional survey analysis. Int J Womens Health. 2017;9:403–11. https://doi.org/10.2147/IJWH.S133212.
AbbVie Inc. Prescribing information for Orlissa (elagolix). North Chicago: AbbVie Inc.; 2017.
Taylor HS, Giudice LC, Lessey BA, Abrao MS, Kotarski J, Archer DF, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40. https://doi.org/10.1056/NEJMoa1700089.
Rubinacci A, Peruzzi E, Modena AB, Zanardi E, Andrei B, De Leo V, et al. Effect of low-dose transdermal E2/NETA on the reduction of postmenopausal bone loss in women. Menopause. 2003;10(3):241–9. https://doi.org/10.1097/00042192-200310030-00012.
Carr BR, Stewart EA, Archer DF, Al-Hendy A, Bradley L, Watts NB, et al. Elagolix alone or with add-back therapy in women with heavy menstrual bleeding and uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;132(5):1252–64. https://doi.org/10.1097/AOG.0000000000002933.
Schlaff WD, Ackerman RT, Al-Hendy A, Archer DF, Barnhart KT, Bradley LD, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382(4):328–40. https://doi.org/10.1056/NEJMoa1904351.
Simon JA, Stewart EA, Owens C, Duan WR, Gao J, Chwalisz K. Elagolix treatment in women with heavy menstrual bleeding-associated with uterine fibroids: efficacy and safety results from a phase 2B study. Fertil Steril. 2017;108(3):e26–7.
Ng J, Chwalisz K, Carter DC, Klein CE. Dose-dependent suppression of gonadotropins and ovarian hormones by elagolix in healthy premenopausal women. J Clin Endocrinol Metab. 2017;102(5):1683–91. https://doi.org/10.1210/jc.2016-3845.
Shebley M, Polepally AR, Nader A, Ng JW, Winzenborg I, Klein CE, et al. Clinical pharmacology of elagolix: an oral gonadotropin-releasing hormone receptor antagonist for endometriosis. Clin Pharmacokinet. 2020;59(3):297–309. https://doi.org/10.1007/s40262-019-00840-7.
Struthers RS, Nicholls AJ, Grundy J, Chen T, Jimenez R, Yen SS, et al. Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix. J Clin Endocrinol Metab. 2009;94(2):545–51. https://doi.org/10.1210/jc.2008-1695.
Chiney MS, Ng J, Gibbs JP, Shebley M. Quantitative assessment of Elagolix enzyme-transporter interplay and drug-drug interactions using physiologically based pharmacokinetic modeling. Clin Pharmacokinet. 2020;59(5):617–27. https://doi.org/10.1007/s40262-019-00833-6.
Elagolix multidiscipline review UFCfDEaR. 2018. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210450Orig1s000MultiD.pdf. Accessed 17 Jan 2020.
Noven Pharmaceuticals Inc. CombiPatch (estradiol/NETA transdermal delivery system) prescribing information. Miami: Noven Pharmaceuticals, Inc.; 2017.
Novo Nordisk Inc. Activella® (estradiol/norethindrone acetate) tablets, for oral use: prescribing information. Plainsboro (NJ): Novo Nordisk Inc.; 2013.
Zhang N, Shon J, Kim MJ, Yu C, Zhang L, Huang SM, et al. Role of CYP3A in oral contraceptives clearance. Clin Transl Sci. 2018;11(3):251–60. https://doi.org/10.1111/cts.12499.
Ng J, Duan WR, Marbury T, Schmidt JM, Klein CE. Elagolix pharmacokinetic profiles in women with renal or hepatic impairment. Clin Pharmacol Drug Dev. 2019;8(8):1053–61. https://doi.org/10.1002/cpdd.640.
Zdravkovic M, Muller M, Larsen S, Degenkolb J, Pabst G. Bioequivalence and relative bioavailability of three estradiol and norethisterone acetate-containing hormone replacement therapy tablets. Int J Clin Pharmacol Ther. 2001;39(1):41–6. https://doi.org/10.5414/cpp39041.
Mueck AO, Seeger H. Smoking, estradiol metabolism and hormone replacement therapy. Curr Med Chem Cardiovasc Hematol Agents. 2005;3(1):45–54. https://doi.org/10.2174/1568016052773270.
Lippert TH, Seeger H, Mueck AO. Estradiol metabolism during oral and transdermal estradiol replacement therapy in postmenopausal women. Horm Metab Res. 1998;30(9):598–600. https://doi.org/10.1055/s-2007-978940.
Kopper NW, Gudeman J, Thompson DJ. Transdermal hormone therapy in postmenopausal women: a review of metabolic effects and drug delivery technologies. Drug Des Dev Ther. 2009;2:193–202. https://doi.org/10.2147/dddt.s4146.
Ansbacher R. The pharmacokinetics and efficacy of different estrogens are not equivalent. Am J Obstet Gynecol. 2001;184(3):255–63. https://doi.org/10.1067/mob.2001.109656.
Balfour JA, Heel RC. Transdermal estradiol: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the treatment of menopausal complaints. Drugs. 1990;40(4):561–82. https://doi.org/10.2165/00003495-199040040-00006.
Ng J, Salem AH, Carter DC, Klein CE, editors. Effects of the co-administration of multiple doses of elagolix on the pharmacokinetics and safety of digoxin in healthy women. In: Annual Meeting of the American College of Clinical Pharmacology; 17-19 September 2017; San Diego (CA).
Kim WY, Benet LZ. P-glycoprotein (P-gp/MDR1)-mediated efflux of sex-steroid hormones and modulation of P-gp expression in vitro. Pharm Res. 2004;21(7):1284–93. https://doi.org/10.1023/b:pham.0000033017.52484.81.
Peng R, Zhang H, Zhang Y, Wei DY. Effects of the ABCB1 (1199G > A) polymorphism on steroid sex hormone-induced P-glycoprotein expression, ATPase activity, and hormone efflux. Med Sci (Basel). 2015;3(4):124–37. https://doi.org/10.3390/medsci3040124.
Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003;144(8):3382–98. https://doi.org/10.1210/en.2003-0192.
Acknowledgments
The authors thank Mia DeFino, MS, ELS, a freelance medical writer under contract with AbbVie for medical writing support and Wesley Wayman, PhD, an AbbVie employee for assistance with the figures.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AN. Data interpretation and manuscript writing was performed by all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
AbbVie funded the studies presented in this article. AbbVie was responsible for the study design, research, analysis, data collection, interpretation of data, and writing, reviewing, and approving of the publication.
Conflicts of Interest/Competing Interests
Ahmed Nader, Nael M. Mostafa, Farah Ali, and Mohamad Shebley are employees of AbbVie, Inc., and may own stocks or options.
Ethics approval
All studies were conducted in accordance with Good Clinical Practice guidelines and the ethical principles that have their origin in the Declaration of Helsinki. The protocols and informed consent forms were approved by the ethics committee or institutional review board at the site.
Consent to participate
All participants provided written informed consent for participation in the studies.
Consent for publication
All authors reviewed and approved the manuscript in the submitted form.
Rights and permissions
About this article
Cite this article
Nader, A., Mostafa, N.M., Ali, F. et al. Drug–Drug Interaction Studies of Elagolix with Oral and Transdermal Low-Dose Hormonal Add-Back Therapy. Clin Pharmacokinet 60, 133–143 (2021). https://doi.org/10.1007/s40262-020-00921-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-020-00921-y