Abstract
Background and Objectives
Two phase I studies assessed the drug–drug interaction potential of apalutamide as a substrate and perpetrator.
Methods
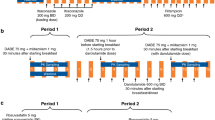
Study A randomized 45 healthy men to single-dose apalutamide 240 mg alone or with strong inhibitors of cytochrome P450 (CYP)3A4 (itraconazole) or CYP2C8 (gemfibrozil). In study B, 23 patients with castration-resistant prostate cancer received probes for CYP3A4 (midazolam), CYP2C9 (warfarin), CYP2C19 (omeprazole), and CYP2C8 (pioglitazone), and transporter substrates for P-glycoprotein (P-gp) (fexofenadine) and breast cancer resistance protein (BCRP)/organic anion transporting polypeptide (OATP) 1B1 (rosuvastatin) at baseline and after repeat once-daily administration of apalutamide 240 mg to steady state.
Results
Systemic exposure (area under the plasma concentration–time curve) to single-dose apalutamide increased 68% with gemfibrozil but was relatively unchanged with itraconazole (study A). Apalutamide reduced systemic exposure to midazolam ↓92%, omeprazole ↓85%, S-warfarin ↓46%, fexofenadine ↓30%, rosuvastatin ↓41%, and pioglitazone ↓18% (study B). After a single dose, apalutamide is predominantly metabolized by CYP2C8, and less by CYP3A4.
Conclusions
Co-administration of apalutamide with CYP3A4, CYP2C19, CYP2C9, P-gp, BCRP or OATP1B1 substrates may cause loss of activity for these medications. Therefore, appropriate mitigation strategies are recommended.




Similar content being viewed by others
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Erleada (apalutamide) [prescribing information]. Horsham (PA): Janssen Pharmaceutical Companies; 2019.
Erleada [summary of product characteristics]. Electronic Medicines Compendium (eMC). https://www.ema.europa.eu/en/documents/product-information/erleada-epar-product-information_en.pdf. Accessed 4 Oct 2019.
Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408–18.
Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13–24.
De Vries R, Jacobs F, Mannens G, Snoeys J, Cuyckens F, Chien C, Ward P. Apalutamide absorption, metabolism, and excretion in healthy men, and enzyme reaction in human hepatocytes. Drug Metab Dispos. 2019;47(5):453–64.
Daily EB, Aquilante CL. Cytochrome P450 2C8 pharmacogenetics: a review of clinical studies. Pharmacogenomics. 2009;10(9):1489–510.
Tian D, Hu Z. CYP3A4-mediated pharmacokinetic interactions in cancer therapy. Curr Drug Metab. 2014;15(8):808–17.
European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) assessment report. https://www.ema.europa.eu/en/documents/assessment-report/erleada-epar-public-assessment-report_en.pdf. Accessed 19 June 2019.
MacLeod AK, McLaughlin LA, Henderson CJ, Wolf CR. Activation status of the pregnane X receptor (PXR) influences vemurafenib availability in humanized mouse models. Cancer Res. 2015;75(21):4573–81.
Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
Honkalammi J, Niemi M, Neuvonen PJ, Backman JT. Gemfibrozil is a strong inactivator of CYP2C8 in very small multiple doses. Clin Pharmacol Ther. 2012;91(5):846–55.
Shimizu M, Uno T, Sugawara K, Tateishi T. Effects of single and multiple doses of itraconazole on the pharmacokinetics of fexofenadine, a substrate of P-glycoprotein. Br J Clin Pharmacol. 2006;62(3):372–6.
Belderbos BPSI, de Wit R, Chien C, et al. An open-label, multicenter, phase Ib study investigating the effect of apalutamide on ventricular repolarization in men with castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2018;82(3):457–68.
Smith MR, Rathkopf DE, Mulders PF, et al. Efficacy and safety of abiraterone acetate in elderly (75 years or older) chemotherapy naïve patients with metastatic castration resistant prostate cancer. J Urol. 2015;194(5):1277–84.
Bonnet C, Boudou-Rouquette P, Azoulay-Rutman E, et al. Potential drug-drug interactions with abiraterone in metastatic castration-resistant prostate cancer patients: a prevalence study in France. Cancer Chemother Pharmacol. 2017;79(5):1051–5.
Jamani R, Lee EK, Berry SR, et al. High prevalence of potential drug–drug interactions in patients with castration-resistant prostate cancer treated with abiraterone acetate. Eur J Clin Pharmacol. 2016;72(11):1391–9.
Hyland R, Osborne T, Payne A, et al. In vitro and in vivo glucuronidation of midazolam in humans. Br J Clin Pharmacol. 2009;67(4):445–54.
Eap CB, Buclin T, Cucchia G, et al. Oral administration of a low dose of midazolam (75 μg) as an in vivo probe for CYP3A activity. Eur J Clin Pharmacol. 2004;60(4):237–46.
Link B, Haschke M, Grignaschi N, et al. Pharmacokinetics of intravenous and oral midazolam in plasma and saliva in humans: usefulness of saliva as matrix for CYP3A phenotyping. Br J Clin Pharmacol. 2008;66(4):473–84.
Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivisto KT. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42(9):819–50.
Andersson T. Pharmacokinetics, metabolism and interactions of acid pump inhibitors. Focus on omeprazole, lansoprazole and pantoprazole. Clin Pharmacokinet. 1996;31(1):9–28.
Andersson T, Miners JO, Veronese ME, Birkett DJ. Identification of human liver cytochrome P450 isoforms mediating secondary omeprazole metabolism. Br J Clin Pharmacol. 1994;37(6):597–604.
Park GJ, Bae SH, Park WS, et al. Drug-drug interaction of microdose and regular-dose omeprazole with a CYP2C19 inhibitor and inducer. Drug Des Dev Ther. 2017;11:1043–53.
Coumadin [prescribing information]. Princeton: Bristol-Myers Squibb Company; 2016.
Kaminsky LS, Zhang ZY. Human P450 metabolism of warfarin. Pharmacol Ther. 1997;73(1):67–74.
Yamazaki H, Shimada T. Human liver cytochrome P450 enzymes involved in the 7-hydroxylation of R- and S-warfarin enantiomers. Biochem Pharmacol. 1997;54(11):1195–203.
Heimark LD, Gibaldi M, Trager WF, O’Reilly RA, Goulart DA. The mechanism of the warfarin-rifampin drug interaction in humans. Clin Pharmacol Ther. 1987;42(4):388–94.
Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27(8):866–71.
Allegra [prescribing information]. Bridgewater: Sanofi-Aventis U.S. LLC; 2007.
Hamman MA, Bruce MA, Haehner-Daniels BD, Hall SD. The effect of rifampin administration on the disposition of fexofenadine. Clin Pharmacol Ther. 2001;69(3):114–21.
Jaakkola T, Laitila J, Neuvonen PJ, Backman JT. Pioglitazone is metabolised by CYP2C8 and CYP3A4 in vitro: potential for interactions with CYP2C8 inhibitors. Basic Clin Pharmacol Toxicol. 2006;99(1):44–51.
Jaakkola T, Backman JT, Neuvonen M, Laitila J, Neuvonen PJ. Effect of rifampicin on the pharmacokinetics of pioglitazone. Br J Clin Pharmacol. 2006;61(1):70–8.
Zhang W, Deng S, Chen XP, et al. Pharmacokinetics of rosuvastatin when coadministered with rifampicin in healthy males: a randomized, single-blind, placebo-controlled, crossover study. Clin Ther. 2008;30(7):1283–9.
Acknowledgements
The authors thank William Turner, PhD, and Ira Mills, PhD, employees of Parexel, for medical writing assistance, which was funded by Janssen Global Services, LLC.
Funding
This study was funded by Janssen Research & Development. Editorial assistance was provided by William Turner, PhD, and Ira Mills, PhD, of Parexel, with funding from Janssen Global Services, LLC.
Author information
Authors and Affiliations
Contributions
Writing [original draft preparation]: ID, JC, IB, PH, AM, PW, JJ, DA, and CC; Writing [review and editing]: ID, JC, IB, PH, AM, PW, JJ, DA, and CC; Research design: PH, AM, JJ, PW, and CC; Performing the research: ID, JC, IB, and DA; Data analysis: PH, AM, JJ, and CC.
Corresponding author
Ethics declarations
Conflict of interest
Ignacio Duran reports personal fees from AstraZeneca, Bayer, Bristol-Myers Squibb, Ipsen, Janssen, MSD, Novartis, Pharmacyclics, Roche Genentech, and Sanofi, non-financial support from AstraZeneca, Ipsen, and Roche Genentech, and grants from AstraZeneca and Roche Genentech outside the submitted work. Joan Carles reports advisory roles for Astellas, Bayer, Bristol-Myers Squibb, Johnson & Johnson, MSD Oncology, Pfizer, Roche, and Sanofi, and speakers’ bureau roles for Asofarma, Astellas, Bayer, and Johnson & Johnson outside the submitted work. Iurie Bulat has no conflicts of interest that are directly relevant to the content of this article. Danielle Armas reports support from Janssen to her employer, Celerion, during the conduct of the study. Peter Hellemans, Anna Mitselos, Peter Ward, James Jiao, and Caly Chien are current or former employees of Janssen Research & Development and hold/held stock in Johnson & Johnson.
Ethics approval
Protocols of both studies were reviewed by an independent ethics committee and institutional review board, and both studies were conducted in accordance with Good Clinical Practice and applicable regulatory requirements.
Consent to participate
Participants in both studies provided written informed consent.
Additional information
Please see the companion article titled “Pharmacokinetic Drug–Drug Interaction of Apalutamide, Part 2: Investigating Interaction Potential Using a Physiologically Based Pharmacokinetic Model.”
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duran, I., Carles, J., Bulat, I. et al. Pharmacokinetic Drug–Drug Interaction of Apalutamide, Part 1: Clinical Studies in Healthy Men and Patients with Castration-Resistant Prostate Cancer. Clin Pharmacokinet 59, 1135–1148 (2020). https://doi.org/10.1007/s40262-020-00882-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-020-00882-2