Abstract
Background and Objectives
Despite limited evidence, cannabidiol-rich cannabis extracts have been popularly used in pediatrics. With increased use, it is critical to determine basic pharmacokinetic parameters of cannabidiol in these extracts in the pediatric population. The objective of this study was to determine the disposition of oral cannabidiol cannabis extracts and drug interactions in children with pediatric epilepsy.
Methods
We conducted a prospective observational study evaluating the disposition of oral cannabidiol in children (< 18 years of age) receiving cannabidiol extracts for epilepsy. Subjects underwent serial blood draws after oral cannabidiol administration. Cannabidiol and metabolites, along with anticonvulsant concentrations were determined.
Results
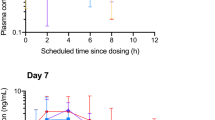
Twenty-nine patients had sufficient pharmacokinetic data and were included in the analysis. Mean age was 9.7 years (standard deviation 4.3) and 17 patients (59%) were male. Median peak plasma cannabidiol concentrations was 13.1 ng/mL (interquartile range 6.8–39.3 ng mL); median time to peak of 2.0 h (interquartile range 2.0–4.0 h). Mean acute elimination half-life of oral cannabidiol was 6.2 h (standard deviation 1.8 h). There was an observed half-life of degradation of 533 days noted for cannabidiol concentrations when stored for 0.6–3.1 years. There was some impact on cannabidiol pharmacokinetic parameters when cannabidiol was co-administered with zonisamide (elimination rate constant and V1) and levetiracetam (elimination rate constant).
Conclusions
In pediatric patients using oral cannabidiol-rich cannabis extract for epilepsy, the time to peak concentration of plasma cannabidiol and average acute elimination half-life were shorter than those reported for adults. Co-administration of zonisamide and levetiracetam had some impact on cannabidiol pharmacokinetic parameters. There was an observed degradation of plasma cannabidiol in long-term storage.
Clinical registration
ClinicalTrials.gov Identifer no. NCT02447198.


Similar content being viewed by others
References
Legal medical marijuana States and DC. 2019. https://medicalmarijuana.procon.org/view.resource.php?resourceID=000881. Accessed 16 Sep 2019.
Izquierdo I, Oshinger OA, Berardi AC. Effect of cannabidiol and of other cannabis sativa compounds on hippocampal seizure discharges. Psychopharmacologia. 1973;28(1):95–102.
Carlini EA, Leite JR, Tennhauser M, Berardi AC. Letter: cannabidiol and cannabis sativa extract protect mice and rats against convulsive agents. J Pharm Pharmacol. 1973;25(8):664–5.
Consroe P, Wolkin A. Cannabidiol-antiepileptic drug comparisons and interactions in experimentally induced seizures in rats. J Pharmacol Exp Ther. 1977;201(1):26–32.
Turkanis SA, Smiley KA, Borys HK, Olsen DM, Karler R. An electrophysiological analysis of the anticonvulsant action of cannabidiol on limbic seizures in conscious rats. Epilepsia. 1979;20(4):351–63.
Turkanis SA, Karler R. Electrophysiology properties of the cannabinoids. J Clin Pharmacol. 1981;21(S1):449S–63S.
Karler R, Turkanis SA. The cannabinoids as potential antiepileptics. J Clin Pharmacol. 1981;21(S1):437S–48S.
Consore P, Benedito MA, Leite JR, Carlini EA, Mechoulam R. Effects of cannabidiol on behavorial seizures cause by convulsant drugs or current in mice. Eur J Pharmacol. 1982;83(3–4):293–8.
Jones NA, Hill AJ, Smith I, Bevan SA, Williams CM. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J Pharmacol Exp Ther. 2010;332(2):569–77.
Hill TD, Cascio MG, Romano B, et al. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br J Pharmacol. 2013;170(3):679–92.
Jones NA, Glyn SE, Aliyama S, et al. Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure. 2012;21(5):344–52.
Colasanti BK, Lindamood C 3rd, Craig CR. Effects of marihuana cannabinoids on seizure activity in cobalt-epileptic rats. Pharmacol Biochem Behav. 1982;16(4):573–8.
Martin AR, Consroe P, Kane VV, et al. Structure-anticonvulsant activity relationship of cannabidiol analogs. NIDA Res Monogr. 1987;79:48–58.
Usami N, Okuda T, Yoshia H, et al. Synthesis and pharmacological evaluation in mice of halogenated cannabidiol derivatives. Chem Pharm Bull (Tokyo). 1999;47(11):1641–5.
Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;3:CD009270.
Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C. Chronic administration of cannabidiol to health volunteers and epileptic patients. Pharmacology. 1980;21(3):175–85.
Carlini EA, Cunha JM. Hypnotic and antiepileptic effects of cannabidiol. J Clin Pharmacol. 1981;21(S1):417S–27S.
Ames FR, Cridland S. Anticonvulsant effect of cannabidiol. S Afr Med J. 1985;69:14.
Mechoulam R, Carlini EA. Toward drugs derived from cannabis. Naturwissenschaften. 1978;65:14–9.
Trembly B, Sherman M. Double-blind clinical study of cannabidiol as a secondary anticonvulsant. In: Marijuana ’90 International Conference on Cannabis and Cannabinoids; 8–11 July 1990; Kolympari; International Association for Cannabinoid Medicines, 1990;Section 2, p. 5.
Porter BE, Jacobson C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. 2013;29(3):574–7.
Maa E, Figi P. The case for medical marijuana in epilepsy. Epilepsia. 2014;55(60):783–6.
CNN. Marijuana stops child’s severe seizures. 2013. http://www.cnn.com/2013/08/07/health/charlotte-child-medical-marijuana. Accessed 1 Oct 2016.
The New York Times. Families see Colorado as new frontier on medical marijuana. 2013. http://www.nytimes.com/2013/12/06/us/families-see-colorado-as-new-frontier-on-medical-marijuana.html?pagewanted=all&_r=0. Accessed 1 Oct 2016.
NPR. Florida Bill would allow medical marijuana for child seizures. 2014. http://www.npr.org/blogs/health/2014/01/16/262481852/florida-bill-would-allow-marijuana-extract-for-child-seizures. Accessed 1 Oct 2016.
Huffington Post. Number of children seeking medical marijuana soars in Colorado. 2014. http://www.huffingtonpost.com/2014/02/13/medical-marijuana-children_n_4768219.html. Accessed 25 Sep 2015.
Colorado Department of Public Health and Environment. Medical marijuana registry program statistics. 2018. https://www.colorado.gov/pacific/sites/default/files/CHED_MMR_Monthly_Report-July-2018.pdf. Accessed 12 Jul 2019.
US FDA. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. 2018. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms. Accessed 12 Jul 2019.
Campbell CT, Phillips MS, Manasco K. Cannabinoids in pediatrics. J Pediatr Pharmcol Ther. 2017;22(3):176–85.
Agurell S, Halldin M, Lingren JE, et al. Pharmacokinetics and metabolism of delta 9-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev. 1986;38:21–42.
Harvey DJ, Mechoulam R. Metabolites of cannabidiol identified in human urine. Xenobiotica. 1990;20:303–20.
Ohhsson A, Lindgren JE, Andersson S, Agurell S, Gillespie H, Hollister LE. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed Environ Mass Spectrom. 1986;13:77–83.
Guy GW, Whitte BA, Robson P. The medicinal uses of cannabis and cannabinoids. Cornwall: TJ International; 2004.
Yamori S, Oakamoto Y, Yamamoto I, Watanabe K. Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP 2D6. Drug Metab Dispos. 2011;39(11):2049–56.
Corroon J, Kight R. Regulatory status of cannabidiol in the United States: a perspective. Cannabis Cannabinoid Res. 2018;3(1):190–4.
Klawitter J, Sempio C, Mörlein S, et al. An atmospheric pressure chemical ionization MS/MS assay using online extraction for the analysis of 11 cannabinoids and metabolites in human plasma and urine. Ther Drug Monit. 2017;39(5):556–64.
Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. 2012;34(4):467–76.
US FDA. Epidiolex (cannabidiol): highlights of prescribing information. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf. Accessed 12 Jul 2019.
Millar SA, Stone NL, Yates AS, O’Sullivan SE. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol. 2018;9:1365.
World Health Organization. Cannabidiol (CBD) pre-review report. 2017. https://www.who.int/medicines/access/controlled-substances/5.2_CBD.pdf. Accessed 16 Sep 2019.
United States Department of Agriculture. Industrial hemp. 2018. https://nifa.usda.gov/industrial-hemp. Accessed 12 Jul 2019.
US FDA. Warning letters and test results for cannabidiol-related products. 2019. https://www.fda.gov/news-events/public-health-focus/warning-letters-and-test-results-cannabidiol-related-products. Accessed 12 Jul 2019.
US FDA. Scientific data and information about products containing cannabis or cannabis-derived compounds; public hearing. 2019. https://www.fda.gov/news-events/fda-meetings-conferences-and-workshops/scientific-data-and-information-about-products-containing-cannabis-or-cannabis-derived-compounds. Accessed 12 Jul 2019.
Chang BS. Cannabidiol and serum antiepileptic drug levels: the ABC’s of CBD with AEDs. Epilepsy Curr. 2018;18(1):33–4.
Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56(8):1246–51.
Wang GS, Bourne DW, Klawitter J, et al. Disposition of oral delta-9 tetrahydrocannabinol (THC) in children receiving cannabis extracts for epilepsy. Clin Toxicol (Phila). 2020;58(2):124–8.
Karchner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral Δ9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem. 2011;57(1):66–75.
Nahler G, Grotenhermen F, Zuardi AW, Crippa JAS. A conversion of oral cannabidiol to delta9-tetrahydrocannabinol seems not to occur in humans. Cannabis Cannabinoid Res. 2017;2(1):81–6.
Nadulski T, Pragst F, Weinberg G, et al. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit. 2005;27(6):799–810.
Scheidweiler KB, Schwope DM, Karchner EL, Desrosier NA, Gorelick DA, Huestis MA. In vitro stability of free and glucuronidated cannabinoids in blood and plasma following controlled smoked cannabis. Clin Chem. 2013;59(7):1108–17.
Taylor L, Crockett J, Tayo B, Morrison G. A phase 1, open-label, parallel-group, single-dose trial of the pharmacokinetics and safety of cannabidiol (CBD) in subjects with mild to severe hepatic impairment. J Clin Pharmacol. 2019;59(8):1110–9.
Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32(11):1053–67.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Colorado Department of Public Health and Environment Medical Marijuana Grant Program (18-103031, to George Sam Wang, David W.A. Bourne, Jost Klawitter, Cristina Sempio, Uwe Christians, Kevin Chapman, Kelly Knupp, Laura Borgelt, Michael F. Wempe, and Lalit Bajaj). George Sam Wang has received royalties from UpToDate, and is a co-investigator on grant NIH1R01DA045051-01A1 on related subjects. Laura Borgelt has received funding for research from the Colorado Department of Public Health and Environment and honoraria for providing continuing education from PharmCon, Inc. on related subjects. Kelly Knupp was a consultant for GW pharmaceuticals and participated in drug safety and monitoring board.
Conflict of interest
George Sam Wang, David W. A. Bourne, Jost Klawitter, Cristina Sempio, Kevin Chapman, Kelly Knupp, Michael F. Wempe, Laura Borgelt, Uwe Christians, Jan Leonard, Kennon Heard, and Lalit Bajaj have no conflicts of interest that are directly relevant to the content of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, G.S., Bourne, D.W.A., Klawitter, J. et al. Disposition of Oral Cannabidiol-Rich Cannabis Extracts in Children with Epilepsy. Clin Pharmacokinet 59, 1005–1012 (2020). https://doi.org/10.1007/s40262-020-00869-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-020-00869-z