Abstract
Background and Objectives
Rituximab is approved in patients with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) and leads to a decrease of ANCA levels. The objectives of this study were to investigate the non-linear pharmacokinetics of rituximab and the relationship between its concentrations and ANCA levels in AAV patients.
Methods
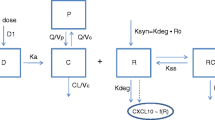
Ninety-two AAV patients from the RAVE (Rituximab in ANCA-Associated Vasculitis) trial were assessed. Both ANCA anti-myeloperoxidase (MPO-ANCA) and anti-proteinase 3 (PR3-ANCA) levels were used as biomarkers. The pharmacokinetics of rituximab were described using a semi-mechanistic two-compartment model that included a latent target antigen turnover and allowed the estimation of specific target-mediated elimination in addition to its non-specific elimination of rituximab. The effect of rituximab on the ANCA level was described using a semi-mechanistic compartment model with a negative feedback (Friberg) model with no transit compartment. A population modeling approach was used.
Results
Our pharmacokinetic and pharmacokinetic–pharmacodynamic (PK-PD) models satisfactorily described both concentration–time and concentration–effect relationship data. The mean (inter-individual standard deviation) estimated non-specific clearance was 0.15 L/day (0.30%) and the target-mediated elimination rate constant was 2.4 × 10−5 nmol/day. The elimination half-lives for MPO-ANCA and PR3-ANCA were 24 and 18 days, respectively.
Conclusions
A non-linear target-mediated elimination of rituximab was detected in AAV patients. Our PK-PD model allowed quantification of the association between rituximab concentrations and ANCA levels. This decrease was deep but delayed, and more sustained in patients with MPO-ANCA than in those with PR3-ANCA. Our results suggest that repeating courses of rituximab might improve the clinical response to rituximab.



Similar content being viewed by others
References
Li J, Levi M, Charoin J-E, Frey N, Kheoh T, Ren S, et al. Rituximab exhibits a long half-life based on a population pharmacokinetic analysis in non-Hodgkin’s lymphoma (NHL) patients. Blood. 2007;110(11):2371.
Rozman S, Grabnar I, Novakovic S, Mrhar A, Jezersek Novakovic B. Population pharmacokinetics of rituximab in patients with diffuse large B-cell lymphoma and association with clinical outcome. Br J Clin Pharmacol. 2017;83(8):1782–90. https://doi.org/10.1111/bcp.13271.
Tout M, Gagez AL, Lepretre S, et al. Influence of FCGR3A-158 V/F genotype and baseline CD20 antigen count on target-mediated elimination of rituximab in patients with chronic lymphocytic leukemia: a study of FILO Group. Clin Pharmacokinet. 2017;56(6):635–47. https://doi.org/10.1007/s40262-016-0470-8.
Tout M, Casasnovas O, Meignan M, et al. Rituximab exposure is influenced by baseline metabolic tumor volume and predicts outcome of DLBCL patients: a Lymphoma Study Association report. Blood. 2017;129(19):2616–23. https://doi.org/10.1182/blood-2016-10-744292.
Regazzi MB, Iacona I, Avanzini MA, et al. Pharmacokinetic behavior of rituximab: a study of different schedules of administration for heterogeneous clinical settings. Ther Drug Monit. 2005;27(6):785–92.
Muller C, Murawski N, Wiesen MH, et al. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood. 2012;119(14):3276–84. https://doi.org/10.1182/blood-2011-09-380949.
Candelaria M, Gonzalez D, Fernandez Gomez FJ, et al. Comparative assessment of pharmacokinetics, and pharmacodynamics between RTXM83, a rituximab biosimilar, and rituximab in diffuse large B-cell lymphoma patients: a population PK model approach. Cancer Chemother Pharmacol. 2018;81(3):515–27. https://doi.org/10.1007/s00280-018-3524-9.
Blasco H, Chatelut E, de Bretagne IB, Congy-Jolivet N, Le Guellec C. Pharmacokinetics of rituximab associated with CHOP chemotherapy in B-cell non-Hodgkin lymphoma. Fundam Clin Pharmacol. 2009;23(5):601–8. https://doi.org/10.1111/j.1472-8206.2009.00714.x.
Ternant D, Henin E, Cartron G, Tod M, Paintaud G, Girard P. Development of a drug-disease simulation model for rituximab in follicular non-Hodgkin’s lymphoma. Br J Clin Pharmacol. 2009;68(4):561–73. https://doi.org/10.1111/j.1365-2125.2009.03494.x.
Ternant D, Cartron G, Henin E, Tod M, Girard P, Paintaud G. Model-based design of rituximab dosage optimization in follicular non-Hodgkin’s lymphoma. Br J Clin Pharmacol. 2012;73(4):597–605. https://doi.org/10.1111/j.1365-2125.2011.04125.x.
Li J, Zhi J, Wenger M, et al. Population pharmacokinetics of rituximab in patients with chronic lymphocytic leukemia. J Clin Pharmacol. 2012;52(12):1918–26. https://doi.org/10.1177/0091270011430506.
Assouline S, Buccheri V, Delmer A, et al. Pharmacokinetics and safety of subcutaneous rituximab plus fludarabine and cyclophosphamide for patients with chronic lymphocytic leukaemia. Br J Clin Pharmacol. 2015;80(5):1001–9. https://doi.org/10.1111/bcp.12662.
Lioger B, Edupuganti SR, Mulleman D, et al. Antigenic burden and serum IgG concentrations influence rituximab pharmacokinetics in rheumatoid arthritis patients. Br J Clin Pharmacol. 2017;83(8):1773–81. https://doi.org/10.1111/bcp.13270.
Ng CM, Bruno R, Combs D, Davies B. Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J Clin Pharmacol. 2005;45(7):792–801. https://doi.org/10.1177/0091270005277075.
Puisset F, White-Koning M, Kamar N, et al. Population pharmacokinetics of rituximab with or without plasmapheresis in kidney patients with antibody-mediated disease. Br J Clin Pharmacol. 2013;76(5):734–40. https://doi.org/10.1111/bcp.12098.
Gota V, Karanam A, Rath S, et al. Population pharmacokinetics of Reditux, a biosimilar Rituximab, in diffuse large B-cell lymphoma. Cancer Chemother Pharmacol. 2016;78(2):353–9. https://doi.org/10.1007/s00280-016-3083-x.
Dayde D, Ternant D, Ohresser M, et al. Tumor burden influences exposure and response to rituximab: pharmacokinetic-pharmacodynamic modeling using a syngeneic bioluminescent murine model expressing human CD20. Blood. 2009;113(16):3765–72. https://doi.org/10.1182/blood-2008-08-175125.
Ternant D, Monjanel H, Venel Y, et al. Nonlinear pharmacokinetics of rituximab in non-Hodgkin lymphomas: a pilot study. Br J Clin Pharmacol. 2019;85(9):2002–10. https://doi.org/10.1111/bcp.13991.
Ternant D, Bejan-Angoulvant T, Passot C, Mulleman D, Paintaud G. Clinical pharmacokinetics and pharmacodynamics of monoclonal antibodies approved to treat rheumatoid arthritis. Clin Pharmacokinet. 2015;54(11):1107–23. https://doi.org/10.1007/s40262-015-0296-9.
Levy G. Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther. 1994;56(3):248–52.
Gibiansky L, Gibiansky E. Target-mediated drug disposition model: approximations, identifiability of model parameters and applications to the population pharmacokinetic-pharmacodynamic modeling of biologics. Expert Opin Drug Metab Toxicol. 2009;5(7):803–12. https://doi.org/10.1517/17425250902992901.
Ternant D, Azzopardi N, Raoul W, Bejan-Angoulvant T, Paintaud G. Influence of antigen mass on the pharmacokinetics of therapeutic antibodies in humans. Clin Pharmacokinet. 2019;58(2):169–87. https://doi.org/10.1007/s40262-018-0680-3.
Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–32. https://doi.org/10.1056/NEJMoa0909905.
Cornec D, Kabat BF, Mills JR, et al. Pharmacokinetics of rituximab and clinical outcomes in patients with anti-neutrophil cytoplasmic antibody associated vasculitis. Rheumatology (Oxford). 2018;57(4):639–50. https://doi.org/10.1093/rheumatology/kex484.
Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. https://doi.org/10.1002/art.37715.
Watts RA, Mahr A, Mohammad AJ, Gatenby P, Basu N, Flores-Suarez LF. Classification, epidemiology and clinical subgrouping of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Nephrol Dial Transpl. 2015;30(Suppl 1):i14–22. https://doi.org/10.1093/ndt/gfv022.
Mahr A, Guillevin L, Poissonnet M, Ayme S. Prevalences of polyarteritis nodosa, microscopic polyangiitis, Wegener’s granulomatosis, and Churg-Strauss syndrome in a French urban multiethnic population in 2000: a capture-recapture estimate. Arthritis Rheum. 2004;51(1):92–9. https://doi.org/10.1002/art.20077.
Cornec D, Cornec-Le Gall E, Fervenza FC, Specks U. ANCA-associated vasculitis—clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol. 2016;12(10):570–9. https://doi.org/10.1038/nrrheum.2016.123.
Fussner LA, Hummel AM, Schroeder DR, et al. Factors determining the clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3. Arthritis Rheumatol. 2016;68(7):1700–10. https://doi.org/10.1002/art.39637.
Specks UMP, Hoffman GS, Langford CA, Spiera R, Seo P, et al. Design of the Rituximab in ANCA-Associated Vasculitis (RAVE) trial. Open Arthritis J. 2011;4:1–18.
Mills JR, Cornec D, Dasari S, et al. Using mass spectrometry to quantify rituximab and perform individualized immunoglobulin phenotyping in ANCA-associated vasculitis. Anal Chem. 2016;88(12):6317–25. https://doi.org/10.1021/acs.analchem.6b00544.
Damoiseaux J, Dahnrich C, Rosemann A, et al. A novel enzyme-linked immunosorbent assay using a mixture of human native and recombinant proteinase-3 significantly improves the diagnostic potential for antineutrophil cytoplasmic antibody-associated vasculitis. Ann Rheum Dis. 2009;68(2):228–33. https://doi.org/10.1136/ard.2007.086579.
Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013;369(5):417–27. https://doi.org/10.1056/NEJMoa1213277.
Wessa P. Free Statistics Software, Office for Research Development and Education, version 1.2.1. 2019. https://www.wessa.net/.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. https://doi.org/10.1159/000180580.
Stone JH, Hoffman GS, Merkel PA, et al. International Network for the Study of the Systemic Vasculitides (INSSYS). A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum. 2001;44(4):912–20. https://doi.org/10.1002/1529-0131(200104)44:4%3c912:AID-ANR148%3e3.0.CO;2-5.
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol. 2002;20(24):4713–21. https://doi.org/10.1200/JCO.2002.02.140.
van Kesteren C, Zandvliet AS, Karlsson MO, et al. Semi-physiological model describing the hematological toxicity of the anti-cancer agent indisulam. Invest New Drugs. 2005;23(3):225–34. https://doi.org/10.1007/s10637-005-6730-3.
Quartino AL, Friberg LE, Karlsson MO. A simultaneous analysis of the time-course of leukocytes and neutrophils following docetaxel administration using a semi-mechanistic myelosuppression model. Invest New Drugs. 2012;30(2):833–45. https://doi.org/10.1007/s10637-010-9603-3.
Brendel K, Comets E, Laffont C, Mentre F. Evaluation of different tests based on observations for external model evaluation of population analyses. J Pharmacokinet Pharmacodyn. 2010;37(1):49–65. https://doi.org/10.1007/s10928-009-9143-7.
RStudio. RStudio: Integrated development environment for R (Version 1.1.383) [Computer software]. Boston, MA; 2015. Retrieved 20 May 2012. Available from http://www.rstudio.org/.
Lavielle M. mlxR: simulation of longitudinal data. R package version 3.2.0. 2017. https://CRAN.R-project.org/package=mlxR.
Melet J, Mulleman D, Goupille P, Ribourtout B, Watier H, Thibault G. Rituximab-induced T cell depletion in patients with rheumatoid arthritis: association with clinical response. Arthritis Rheum. 2013;65(11):2783–90. https://doi.org/10.1002/art.38107.
Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236:125–38. https://doi.org/10.1111/j.1600-065X.2010.00912.x.
Waldmann TA, Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110.
Scheiermann N, Kuwert EK. Uptake and elimination of hepatitis B immunoglobulins after intramuscular application in man. Dev Biol Stand. 1983;54:347–55.
Thai LH, Charles P, Resche-Rigon M, Desseaux K, Guillevin L. Are anti-proteinase-3 ANCA a useful marker of granulomatosis with polyangiitis (Wegener’s) relapses? Results of a retrospective study on 126 patients. Autoimmun Rev. 2014;13(3):313–8. https://doi.org/10.1016/j.autrev.2013.11.003.
National Institute of Allergy and Infectious Diseases (NIAID). Rituximab for the treatment of Wegener’s granulomatosis and microscopic polyangiitis (RAVE) [ClinicalTrials.gov identifier NCT00104299]. National Institutes of Health, ClinicalTrials.gov. https://clinicaltrials.gov. Accessed 24 Sep 2019.
Acknowledgements
The authors thank Melissa Cheu, Melissa R. Snyder, Amber M. Hummel, Carol A. Langford, Peter A. Merkel, Paul A. Monach, Philip Seo, Robert F. Spiera, E. William St Clair, Brian F. Kabat, John R. Mills, Darrell R. Schroeder, Matthew D. Cascino, Paul Brunetta, David L. Murray, Fernando Fervenza, and David R. Barnidge for acquiring the data. The authors thank John H. Stone, the principal investigator of the clinical study, for designing the study and acquiring the data.
Author information
Authors and Affiliations
Contributions
AB analyzed and interpreted the data, and wrote the manuscript. DM initiated the study, participated in data interpretation, and reviewed the manuscript. US initiated the study, acquired the data, participated in data interpretation, and reviewed the manuscript. VG and GP proposed data investigation methods, participated in data interpretation, and reviewed the manuscript. NA participated in data interpretation and reviewed the manuscript. DC acquired the data, participated in data interpretation, and reviewed the manuscript. DT supervised data analysis and interpretation, manuscript redaction, and reviewed the manuscript.
Corresponding author
Ethics declarations
Funding
The RAVE (Rituximab in ANCA-Associated Vasculitis) trial, from which these data were obtained, was supported by a grant from the National Institute of Allergy and Infectious Diseases to the Immune Tolerance Network (grant N01-AI-15416; protocol no. ITN021AI). Genentech, Inc. and Biogen IDEC, Inc. provided the study medications and partial funding for the trial. Genentech, Inc. performed the rituximab concentration measurements by ELISA for the trial. The ANCA measurements by ELISA were performed at the Mayo Clinic using test kits provided by Euroimmun, Inc. Divi Cornec received fellowship grants from the French Society of Rheumatology and from Brest University Hospital, France. Ulrich Speck’s laboratory is supported by funds from the Connor Group Foundation and the Mayo Foundation. This work was partly supported by the French Higher Education and Research Ministry under the program ‘Investissements d’avenir’ (grant agreement: LabEx MAbImprove ANR-10-LABX-53-01).
Conflict of interest
Amina Bensalem, Nicolas Azzopardi, Valérie Gouilleux-Gruart, Divi Cornec, and Ulrich Specks have no conflicts of interest to declare. Denis Mulleman reports grants from Abbvie and Nordic Pharma, and consultancy fees from MSD, Novartis, UCB, and Pfizer. Gilles Paintaud reports grants received by his research team from Novartis, Roche Pharma, Genzyme, MSD, Chugai, and Pfizer. David Ternant reports lecture fees from Novartis, Amgen, and Boehringer Ingelheim.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bensalem, A., Mulleman, D., Paintaud, G. et al. Non-Linear Rituximab Pharmacokinetics and Complex Relationship between Rituximab Concentrations and Anti-Neutrophil Cytoplasmic Antibodies (ANCA) in ANCA-Associated Vasculitis: The RAVE Trial Revisited. Clin Pharmacokinet 59, 519–530 (2020). https://doi.org/10.1007/s40262-019-00826-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-019-00826-5