Abstract
Background
Venetoclax is a selective inhibitor of B-cell lymphoma-2, which plays a role in the development of various autoimmune diseases including systemic lupus erythematosus. The aim of these analyses was to quantify the exposure-response relationship for venetoclax effects on B-lymphocyte and total lymphocyte counts as pharmacodynamic markers of efficacy and safety, respectively, in women with systemic lupus erythematosus. The developed modeling framework was also used to evaluate venetoclax effects following cyclic, continuous, or induction/maintenance dosing paradigms as potential dosing alternatives in systemic lupus erythematosus.
Methods
Serial pharmacokinetic and lymphocyte count data from 73 women enrolled in a phase I study of venetoclax (single doses of 10–500 mg or two cycles of 30–600 mg or placebo once daily for 7 days followed by a 21-day washout) were analyzed using a sequential population pharmacokinetic/pharmacodynamic modeling approach. Simulations to evaluate changes in B-lymphocyte and total lymphocyte counts following different venetoclax dosing scenarios were conducted.
Results
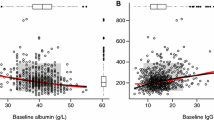
Effect of venetoclax plasma exposures on B lymphocytes was described using an indirect linear response model and on total lymphocytes using a maximal response (Emax) with an effect site compartment. Baseline lymphocyte counts were significant covariates on the slope and half maximal inhibitory concentration parameter estimates for the respective models; with higher baseline counts associated with a greater reduction upon treatment with venetoclax. Model simulations showed that continuous dosing with lower doses of venetoclax (e.g., 150 mg daily) are predicted to achieve similar maximal effects on B-lymphocyte counts compared to cyclic dosing with higher doses (e.g., 400 mg 1 week on/3 weeks off); with better recovery of total lymphocyte counts during off-treatment weeks for the cyclic regimens.
Conclusions
Venetoclax treatment in women with systemic lupus erythematosus was associated with exposure-dependent reductions in B lymphocytes, and to a lesser extent, total lymphocyte counts. Results from this study support evaluation of B-cell lymphoma-2 inhibitors as potential therapies for the treatment of systemic lupus erythematosus.
Clinicaltrials.gov
NCT01686555.





Similar content being viewed by others
References
Wahren-Herlenius M, Dorner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet. 2013;382:819–31.
Pozsgay J, Szekanecz Z, Sarmay G. Antigen-specific immunotherapies in rheumatic diseases. Nat Rev Rheumatol. 2017;13:525–37.
Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;384:1878–88.
Margery-Muir AA, Bundell C, Nelson D, Groth DM, Wetherall JD. Gender balance in patients with systemic lupus erythematosus. Autoimmun Rev. 2017;16:258–68.
Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12:716–30.
Liphaus BL, Kiss MH. The role of apoptosis proteins and complement components in the etiopathogenesis of systemic lupus erythematosus. Clinics (Sao Paulo). 2010;65:327–33.
Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, et al. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci USA. 1991;88:8661–5.
Gatenby PA, Irvine M. The bcl-2 proto-oncogene is overexpressed in systemic lupus erythematosus. J Autoimmun. 1994;7:623–31.
Liphaus BL, Kiss MH, Carrasco S, Goldenstein-Schainberg C. Increased Fas and Bcl-2 expression on peripheral mononuclear cells from patients with active juvenile-onset systemic lupus erythematosus. J Rheumatol. 2007;34:1580–4.
Venclexta (venetoclax) [US prescribing information]. North Chicago (IL): AbbVie Inc.; 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208573s009lbl.pdf. Accessed 28 Aug 2019.
Zhan Y, Carrington EM, Ko HJ, Vikstrom IB, Oon S, Zhang JG, et al. Bcl-2 antagonists kill plasmacytoid dendritic cells from lupus-prone mice and dampen interferon-alpha production. Arthritis Rheumatol. 2015;67:797–808.
Wang LC. ABT-199, a potent and selective BCL-2 inhibitor, prevents lupus nephritis in the spontaneous NZB/W F1 mouse model by depleting selective lymphocyte populations while sparing platelets. Arthritis Rheumatol. 2015;67(Suppl 10): San Francisco, CA2015.
Ko K, Wang J, Perper S, Jiang Y, Yanez D, Kaverina N, et al. Bcl-2 as a therapeutic target in human tubulointerstitial inflammation. Arthritis Rheumatol. 2016;68:2740–51.
Wang LC. Lymphocyte depletion, recovery and efficacy in NZBWF1 lupus mice following continuous or intermittent dosing regimen of venetoclax (ABT-199), a potent and selective BCL-2 inhibitor. Arthritis Rheumatol. 2015;67:2163–4 (San Francisco, CA2015).
Lu P, Fleischmann R, Curtis C, Ignatenko S, Clarke SH, Desai M, et al. Safety and pharmacodynamics of venetoclax (ABT-199) in a randomized single and multiple ascending dose study in women with systemic lupus erythematosus. Lupus. 2017;27:290–302.
Minocha M, Zeng J, Medema JK, Othman AA. Pharmacokinetics of the B-cell lymphoma 2 (Bcl-2) inhibitor venetoclax in female subjects with systemic lupus erythematosus. Clin Pharmacokinet. 2018;57:1185–98.
Salem AH, Hu B, Freise KJ, Agarwal SK, Sidhu DS, Wong SL. Evaluation of the pharmacokinetic interaction between venetoclax, a selective BCL-2 inhibitor, and warfarin in healthy volunteers. Clin Drug Investig. 2017;37:303–9.
Pisetsky DS. Anti-DNA and autoantibodies. Curr Opin Rheumatol. 2000;12:364–8.
Nashi E, Wang Y, Diamond B. The role of B cells in lupus pathogenesis. Int J Biochem Cell Biol. 2010;42:543–50.
Anolik JH. B cell biology and dysfunction in SLE. Bull NYU Hosp Jt Dis. 2007;65:182–6.
Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–37.
Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–81.
Jayne D. Role of rituximab therapy in glomerulonephritis. J Am Soc Nephrol. 2010;21:14–7.
Sanz I, Lee FE. B cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:326–37.
Khare SD, Sarosi I, Xia XZ, McCabe S, Miner K, Solovyev I, et al. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci USA. 2000;97:3370–5.
Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–9.
Jacobi AM, Huang W, Wang T, Freimuth W, Sanz I, Furie R, et al. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2010;62:201–10.
Regola F, Piantoni S, Reggia R, Kumar R, Andreoli L, Franceschini F, et al. B and T lymphocytes modifications after belimumab treatment in patients with systemic lupus erythematosus. Ann Rheum Dis. 2018;77(1415):1–5. https://doi.org/10.1136/annrheumdis-2018-eular.3150.
Dascalu C, Davidson A, Mackay MC, Furie R, Huang W, Aranow C. The effect of belimumab on peripheral blood cells in patients with systemic lupus erythematosus [abstract]. Arthritis Rheumatol. 2015;67(Suppl. 10). https://acrabstracts.org/abstract/the-effect-of-belimumab-on-peripheral-blood-cells-in-patients-with-systemic-lupus-erythematosus/. Accessed 9 May 2019.
Grebe K, Perper S, O’Connor L, Schwartz A, Goess C, Hartman D, et al. Venetoclax (ABT-199), a potent and selective BCL-2 inhibitor, is efficacious in NZB/WF1 mouse model of lupus nephritis and reduces human lymphocyte lifespan in vitro (BA4P.127). J Immunol. 2015;194(1 Suppl.):47.7. http://www.jimmunol.org/content/194/1_Supplement/47.7. Accessed 9 May 2019.
Freise KJ, Jones AK, Eckert D, Mensing S, Wong SL, Humerickhouse RA, et al. Impact of venetoclax exposure on clinical efficacy and safety in patients with relapsed or refractory chronic lymphocytic leukemia. Clin Pharmacokinet. 2017;56:515–23.
Acknowledgments
Medical writing support was provided by Wesley Wayman, an employee of AbbVie.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was sponsored by AbbVie. AbbVie contributed to the study design, research, and interpretation of data, and the writing, review, and approval of the publication.
Conflict of Interest
Ahmed Nader and Ahmed A. Othman are employees of AbbVie. Mukul Minocha is a former employee of AbbVie. All authors may hold AbbVie stock or stock options.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standard.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Data Sharing
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), provided the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nader, A., Minocha, M. & Othman, A.A. Exposure-Response Analyses of the Effects of Venetoclax, a Selective BCL-2 Inhibitor, on B-Lymphocyte and Total Lymphocyte Counts in Women with Systemic Lupus Erythematosus. Clin Pharmacokinet 59, 335–347 (2020). https://doi.org/10.1007/s40262-019-00818-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-019-00818-5