Abstract
Background and Aims
In this study, we evaluate the performance of allometric concepts to predict the implications of age and size on the pharmacokinetics of lamotrigine, and assess the dose rationale across different age groups from 0.2 to 91 years.
Methods
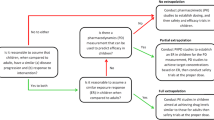
An allometrically scaled pharmacokinetic model was developed using adolescent and adult data, taking into account the effect of comedications. Model parameters were then used to extrapolate lamotrigine pharmacokinetics to older adults (> 65 years), children (4–12 years) and infants and toddlers (0.2–2.0 years). In addition, simulations were performed to identify the implication of different doses and dosing regimens for each population, so as to ensure steady-state concentrations within a predefined reference range.
Results
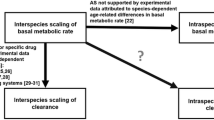
The pharmacokinetics of lamotrigine was best described using a one-compartment model with first-order absorption and elimination. Carbamazepine, phenytoin, and valproic acid changed systemic clearance (CL) by + 76.5, + 129, and − 47.4%, respectively. Allometric principles allowed accurate extrapolation of disposition parameters to older adults and children older than 4 years of age. A maturation function was required to describe changes in exposure in younger patients. Compared with adults, a child aged 1.7 years has a 31.5% higher CL, after correcting for body weight. Patients > 65 years of age showed a decrease in CL of approximately 15%.
Conclusion
Population pharmacokinetic models are usually limited to a subgroup of patients, which may mask the identification of factors contributing to interindividual variability. The availability of an integrated model including the whole patient population provides insight into the role of age-related changes in the disposition of lamotrigine, and potential implications for maintenance dose optimisation in any future trials.
Trial Registration
According to GlaxoSmithKline’s Clinical Trial Register, data from the GlaxoSmithKline studies LAM100034 and LEP103944, corresponding to ClinicalTrials.gov identifiers NCT00113165 and NCT00264615, used in this work, have been used in previous publications (doi: https://doi.org/10.1212/01.wnl.0000277698.33743.8b, https://doi.org/10.1111/j.1528-1167.2007.01274.x).







Similar content being viewed by others
Change history
25 April 2018
Effect of Age-Related Factors on the Pharmacokinetics of Lamotrigine and Potential Implications for Dose Optimisation in Epilepsy Patients should read.
References
Steiner TJ, Dellaportas CI, Findley LJ, Gross M, Gibberd FB, Perkin GD, Park DM, Abbott R. Lamotrigine monotherapy in newly diagnosed untreated epilepsy: a double-blind comparison with phenytoin. Epilepsia. 1999;40(5):601–7.
Mullens EL. Clinical experience with lamotrigine monotherapy in adults with newly diagnosed epilepsy: a review of published randomised clinical trials. Clin Drug Investig. 1998;16(2):125–33.
Fitton A, Goa KL. Lamotrigine: An update of its pharmacology and therapeutic use in epilepsy. Drugs. 1995;50(4):691–713.
Brodie MJ, Richens A, Yuen A, UK, Lamotrigine/Carbamazepine Monotherapy Trial Group. Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. Lancet. 1995;345(8948):476–9.
Italiano D, Perucca E. Clinical pharmacokinetics of new-generation antiepileptic drugs at the extremes of age: an update. Clin Pharmacokinet. 2013;52(8):627–45.
Anderson GD, Saneto RP. Modified-release formulations of second-generation antiepileptic drugs: pharmacokinetic and clinical aspects. CNS Drugs. 2015;29(8):669–81.
Lovrić M, Božina N, Hajnšek S, Kuzman MR, Sporiš D, Lalić Z, et al. Association between lamotrigine concentrations and ABCB1 polymorphisms in patients with epilepsy. Ther Drug Monit. 2012;34(5):518–25.
Landmark CJ, Baftiu A, Tysse I, Valsø B, Larsson PG, Rytter E, Johannessen SI. Pharmacokinetic variability of four newer antiepileptic drugs, lamotrigine, levetiracetam, oxcarbazepine, and topiramate. Ther Drug Monit. 2012;34(4):1.
Ohman I, Vitols S, Tomson T. Lamotrigine in pregnancy: pharmacokinetics during delivery, in the neonate, and during lactation. Epilepsia. 2000;41(6):709–13.
Pennell PB, Peng L, Newport DJ, Ritchie JC, Koganti A, Holley DK, et al. Lamotrigine in pregnancy: clearance, therapeutic drug monitoring, and seizure frequency. Neurology. 2008;70(22):2130–6.
Chen H, Yang K, Choi S, Fischer JH, Jeong H. Up-regulation of UDP-glucuronosyltransferase (UGT) 1A4 by 17beta-estradiol: a potential mechanism of increased lamotrigine elimination in pregnancy. Drug Metab Dispos. 2009;37(9):1841–7.
Fotopoulou C, Kretz R, Bauer S, Schefold JC, Schmitz B, Dudenhausen JW, et al. Prospectively assessed changes in lamotrigine-concentration in women with epilepsy during pregnancy, lactation and the neonatal period. Epilepsy Res. 2009;85(1):60–4.
Pirie DAJ, Al Wattar BH, Pirie AM, Houston V, Siddiqua A, Doug M, et al. Effects of monitoring strategies on seizures in pregnant women on lamotrigine: A meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;172(1):26–31.
Chen C. Validation of a population pharmacokinetic model for adjunctive lamotrigine therapy in children. Br J Clin Pharmacol. 2000;50(2):135–45.
Gidal BE, Anderson GD, Rutecki PR, Shaw R, Lanning A. Lack of an effect of valproate concentration on lamotrigine pharmacokinetics in developmentally disabled patients with epilepsy. Epilepsy Res. 2000;42(1):23–31.
Reimers A, Skogvoll E, Sund JK, Spigset O. Lamotrigine in children and adolescents: the impact of age on its serum concentrations and on the extent of drug interactions. Eur J Clin Pharmacol. 2007;63(7):687–92.
He D, Wang L, Qin J, Zhang S, Lu W, Li L, et al. Population pharmacokinetics of lamotrigine in Chinese children with epilepsy. Acta Pharmacol Sin. 2012;33(11):1417–23.
Brzaković B, Vučićević K, Kovačević SV, Miljković B, Prostran M, Martinović Ž, et al. Pharmacokinetics of lamotrigine in paediatric and young adult epileptic patients–nonlinear mixed effects modelling approach. Eur J Clin Pharmacol. 2014;70(2):179–85.
Baldoni AO, Freitas-Lima P, de Santi Ferreira FI, Martinez EZ, Queiroz RHC, Sakamoto AC, et al. An investigation of the influence of patient-related factors and comedications on lamotrigine clearance in patients with epilepsy. Clin Exp Pharmacol Physiol. 2016;43(7):685–9.
Zhang Z, Ji S, Han Y, Zang L, Wang Y. Population pharmacokinetic models of lamotrigine in different age groups of Chinese children with epilepsy. Eur J Clin Pharmacol. 2017;73:445–53.
Reimers A, Skogvoll E, Sund JK, Spigset O. Drug interactions between lamotrigine and psychoactive drugs: evidence from a therapeutic drug monitoring service. J Clin Psychopharmacol. 2005;25:342–8.
Lott R. Lamotrigine: treatment of epilepsy in the elderly. Consult Pharm. 2003;18(11):979–92.
Punyawudho B, Ramsay RE, Macias FM, Rowan AJ, Collins JF, Brundage RC, et al. Population pharmacokinetics of lamotrigine in elderly patients. J Clin Pharmacol. 2008;48(4):455–63.
Polepally AR, Remmel RP, Brundage RC, Leppik IE, Rarick JO, Ramsay RE, et al. Steady-state pharmacokinetics and bioavailability of immediate-release and extended-release formulations of lamotrigine in elderly epilepsy patients: use of stable isotope methodology. J Clin Pharmacol. 2015;55(10):1101–8.
Tomson T. Gender aspects of pharmacokinetics of new and old AEDs: pregnancy and breast-feeding. Ther Drug Monit. 2005;27:718–21.
Wegner I, Wilhelm AJ, Lambrechts DAJE, Sander JW, Lindhout D. Effect of oral contraceptives on lamotrigine levels depends on comedication. Acta Neurol Scand. 2014;129(6):393–8.
Wegner I, Wilhelm AJ, Sander JW, Lindhout D. The impact of age on lamotrigine and oxcarbazepine kinetics: a historical cohort study. Epilepsy Behav. 2013;29(1):217–21.
Mallaysamy S, Johnson MG, Rao PGM, Rajakannan T, Bathala L, Arumugam K, et al. Population pharmacokinetics of lamotrigine in Indian epileptic patients. Eur J Clin Pharmacol. 2013;69(1):43–52.
Hussein Z, Posner J. Population pharmacokinetics of lamotrigine monotherapy in patients with epilepsy: retrospective analysis of routine monitoring data. Br J Clin Pharmacol. 1997;43(5):457–65.
Chan V, Morris RG, Ilett KF, Tett SE. Population pharmacokinetics of lamotrigine. Ther Drug Monit. 2001;23(6):630–5.
Singkham N, Towanabut S, Lertkachatarn S, Punyawudho B. Influence of the UGT2B7-161C-T polymorphism on the population pharmacokinetics of lamotrigine in Thai patients. Eur J Clin Pharmacol. 2013;69(6):1285–91.
Holford N, Heo Y, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102(9):2941–52.
van Dijkman SC, Alvarez-Jimenez R, Danhof M, Della Pasqua O. Pharmacotherapy in pediatric epilepsy: from trial and error to rational drug and dose selection – a long way to go. Expert Opinion Drug Metab Toxicol. 2016;12(10):1143–56.
Wolf P. Lamotrigine: preliminary clinical observations on pharmacokinetics and interactions with traditional antiepileptic drugs. J Epilepsy. 1992;5(2):73–9.
Piña-Garza JE, Levisohn P, Gucuyener K, Mikati MA, Warnock CR, Conklin HS, et al. Adjunctive lamotrigine for partial seizures in patients aged 1 to 24 months. Neurology. 2008;70(22 Pt 2):2099–108.
Pellock JM, Carman WJ, Thyagarajan V, Daniels T, Morris DL, D’Cruz O. Efficacy of antiepileptic drugs in adults predicts efficacy in children: a systematic review. Neurology. 2012;79(14):1482–9.
Bauer RJ. NONMEM user guide introduction to NONMEM 7.3.0. Hanover: ICON Development Solutions; 2014.
Lindbom L, Pihlgren P, Jonsson N. PsN-Toolkit: a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–57.
Keizer RJ, van Benten M, Beijnen JH, Schellens JHM, Huitema ADR. Piraña and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Programs Biomed. 2011;101(1):72–9.
Keizer RJ, Karlsson MO, Hooker A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacomet Syst Pharmacol. 2013;2:e50.
R Development Core Team. R: a language and environment for statistical computing. Vienna: The R Foundation for Statistical Computing; 2011.
de Onis M, The WHO. Child growth standards. World Rev Nutr Diet. 2015;113:278–94.
Luscombe M, Owens B. Weight estimation in resuscitation: is the current formula still valid? Arch Dis Child. 2007;92(5):412–5.
Patsalos PN, Berry DJ, Bourgeois BFD, Cloyd JC, Glauser TA, Johannessen SI, et al. Antiepileptic drugs—best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring. ILAE Commission on Therapeutic Strategies. Epilepsia. 2008;49(7):1239–76.
Liu L, Zhao L, Wang Q, Qiu F, Wu X, Ma Y. Influence of valproic acid concentration and polymorphism of UGT1A4∗3, UGT2B7 -161C > T and UGT2B7∗2 on serum concentration of lamotrigine in Chinese epileptic children. Eur J Clin Pharmacol. 2015;71(11):1341–7.
Milosheska D, Lorber B, Vovk T, Kastelic M, Dolžan V, Grabnar I. Pharmacokinetics of lamotrigine and its metabolite N-2-glucuronide: influence of polymorphism of UDP-glucuronosyltransferases and drug transporters. Br J Clin Pharmacol. 2016;82:399–411.
Rivas N, Buelga DS, Elger CE, Santos-Borbujo J, Otero MJ, Domínguez-Gil A, et al. Population pharmacokinetics of lamotrigine with data from therapeutic drug monitoring in German and Spanish patients with epilepsy. Ther Drug Monit. 2008;30(4):483–9.
Almeida AM, Falcão AC, Sales F, Baldeiras I, Rocha MJ, Caramona MM. Lamotrigine pharmacokinetic evaluation in epileptic patients submitted to VEEG monitoring. Eur J Clin Pharmacol. 2006;62(9):737–42.
Lardizabal DV, Morris HH, Hovinga CA, Mar Del Carreño M. Tolerability and pharmacokinetics of oral loading with lamotrigine in epilepsy monitoring units. Epilepsia. 2003;44(4):536–9.
Grasela TH, Fiedler-Kelly J, Cox E, Womble GP, Risner ME, Chen C. Population pharmacokinetics of lamotrigine adjunctive therapy in adults with epilepsy. Pharmacokinet Pharmacodyn. 1999;39(4):373–84.
Ramsay RE, Pellock JM, Garnett WR, Sanchez RM, Valakas AM, Wargin WA, et al. Pharmacokinetics and safety of lamotrigine (Lamictal) in patients with epilepsy. Epilepsy Res. 1991;10:191–200.
Anderson BJ, Holford NHG. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25–36.
Cella M, Knibbe C, de Wildt SN, Van Gerven J, Danhof M, Della Pasqua O. Scaling of pharmacokinetics across paediatric populations: the lack of interpolative power of allometric models. Br J Clin Pharmacol. 2012;74(3):525–35.
Piana C, Danhof M, Della Pasqua O. Influence of covariate distribution on the predictive performance of pharmacokinetic models in paediatric research. Br J Clin Pharmacol. 2014;78(1):145–57.
Weintraub D, Buchsbaum R, Resor S, Hirsch L. Effect of antiepileptic drug comedication on lamotrigine clearance. Arch Neurol. 2005;62(9):1432–6.
Arif H, Svoronos A, Resor SR Jr, Buchsbaum R, Hirsch LJ. The effect of age and comedication on lamotrigine clearance, tolerability, and efficacy. Epilepsia. 2011;52(10):1905–13.
Kim H-J, Kim T-E, Joo EY, Seo D-W, Lee S-Y, Hong SB. Effect of comedication on lamotrigine clearance in Korean epilepsy patients. Clin Chim Acta. 2015;2014(438):269–73.
Lamotrigine (Rx). Lamictal, Lamictal XR, Lamictal ODT: Dosing and uses. http://reference.medscape.com/drug/lamictal-lamotrigine-343012. Accessed 25 Sept 2017.
Miyagi SJ, Collier AC. Pediatric development of glucuronidation: the ontogeny of hepatic UGT1A4. Drug Metab Dispos. 2007;35(9):1587–92.
Strassburg CP, Strassburg A, Kneip S, Barut A, Tukey RH, Rodeck B, et al. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut. 2002;50:259–65.
Krekels EHJ, Johnson TN, den Hoedt SM, Rostami-Hodjegan A, Danhof M, Tibboel D, et al. From pediatric covariate model to semiphysiological function for maturation: part II—sensitivity to physiological and physicochemical properties. CPT Pharmacomet Syst Pharmacol. 2012;1(10):e10.
van Dijkman SC, Rauwé WM, Danhof M, Della Pasqua O. Pharmacokinetic interactions and dosing rationale for antiepileptic drugs in adults and children. Br J Clin Pharmacol. 2017;. https://doi.org/10.1111/bcp.13400 (Epub 16 Aug 2017).
van Dijkman SC, Wicha SG, Danhof M, Della Pasqua OE. Individualized dosing algorithms and therapeutic monitoring for antiepileptic drugs. Clin Pharmacol Ther. 2017;. https://doi.org/10.1002/cpt.777 (Epub 27 Jun 2017).
Acknowledgements
The authors thank Khilit Shah for his assistance in retrieving the clinical study data, and Corine Visser for her editorial support.
Author information
Authors and Affiliations
Contributions
Sven van Dijkman, Nico de Jager and Willem Rauwé performed the data analysis; Sven van Dijkman and Oscar Della Pasqua wrote the manuscript; and Meindert Danhof and Oscar Della Pasqua coordinated the investigations and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Sven van Dijkman had support from the Global Research in Paediatrics consortium (GRiP). In addition to his role in GRiP, Oscar Della Pasqua is also Senior Director, Clinical Pharmacology, at GlaxoSmithKline. Nico C. B. de Jager, Willem M. Rauwé and Meindert Danhof declare no conflicts of interest.
Ethical Approval
The research presented in this paper was based on already existing data. The data used were derived from clinical trials performed by GlaxoSmithKline, which were all performed according to the Declaration of Helsinki and any additional ethical and practical standards applicable at the local trial sites.
Funding
The research leading to these results has received funding from the European Union Seventh Framework Programme FP7/2007-2013 under Grant agreement no. 261060.
Additional information
The original version of this article was revised as per the corrections listed in the following: https://doi.org/10.1007/s40262-018-0660-7.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Dijkman, S.C., de Jager, N.C.B., Rauwé, W.M. et al. Effect of Age-Related Factors on the Pharmacokinetics of Lamotrigine and Potential Implications for Maintenance Dose Optimisation in Future Clinical Trials. Clin Pharmacokinet 57, 1039–1053 (2018). https://doi.org/10.1007/s40262-017-0614-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0614-5