Abstract
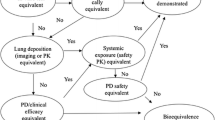
In recent years, pathways for the development and approval of bioequivalent inhaled products have been established for regulated markets, including the European Union (EU), and a number of orally inhaled products (OIPs) have been approved in the EU solely on the basis of in vitro and pharmacokinetic data. This review describes how these development pathways are structured and their implications for the treatment of airway diseases such as asthma. The EU guidance follows a stepwise approach that includes in vitro criteria as the first step. If all in vitro criteria are not met, the second step is based on pharmacokinetic evaluations, which include assessments of lung and systemic bioavailability. If all pharmacokinetic criteria are not met, the third step is based on clinical endpoint studies. In this review, the scientific rationale of the European Medicines Agency guidance for the development of bioequivalent OIPs is reviewed with the focus on the development of bioequivalent OIPs in the EU. Indeed, we discuss the advantages and disadvantages of the weight-of-evidence and stepwise approaches. The evidence indicates that the EU guidance is robust and, unlike clinical endpoint studies, the pharmacokinetic studies are far more sensitive to measure the minor differences, i.e. deposition and absorption rates, in drug delivery from the test and reference products and, thus, should be best suited for assessing bioequivalence. The acceptance range of the 90% confidence intervals for pharmacokinetic bioequivalence (i.e. 80–125% for both the area under the plasma concentration–time curve and maximum plasma concentration) represent appropriately conservative margins for ensuring equivalent safety and efficacy of the test and reference products.








Similar content being viewed by others
References
Global Initiative for Asthma. Global strategy for asthma management and prevention; 2015. http://ginasthma.org/wp-content/uploads/2016/01/GINA_Pocket_2015.pdf. Accessed 23 Feb 2016.
Gergen PJ. Understanding the economic burden of asthma. J Allergy Clin Immunol. 2001;107(5 Suppl):S445–8.
Morgan M, Khan DA. Asthma: epidemiology, burden and quality of life. Adv Psychosom Med. 2003;24:1–15.
Sennhauser FH, Braun-Fahrlander C, Wildhaber JH. The burden of asthma in children: a European perspective. Paediatr Respir Rev. 2005;6:2–7.
Woolcock AJ, Bastiampillai SA, Marks GB, Keena VA. The burden of asthma in Australia. Med J Aust. 2001;175:141–5.
Barnett SBL, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127:145–52.
Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, et al. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;19:24.
Committee for Medicinal Products for Human Use (CHMP). Guideline on the requirements for clinical documentation for orally inhaled products (OIP) including the requirements for demonstration of therapeutic equivalence between two inhaled products for use in the treatment of asthma and chronic obstructive pulmonary disease (COPD) in adults and for use in the treatment of asthma in children and adolescents. CPMP/EWP/4151/00/Rev. 1; January 2009. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003504.pdf. Accessed 23 Feb 2016.
Lee SL, Saluja B, Garcia-Arieta A, Santos GML, Li Y, Lu S, et al. Regulatory considerations for approval of generic inhalation drug products in the US, EU, Brazil, China, and India. AAPS J. 2015;17(5):1285–304.
Daley-Yates PT, Parkins DA, Thomas MJ, Gillett B, House KW, Ortega HG. Pharmacokinetic, pharmacodynamic, efficacy, and safety data from two randomized, double-blind studies in patients with asthma and an in vitro study comparing two dry-powder inhalers delivering a combination of salmeterol 50 mcg and fluticasone propionate 250 mcg: Implications for establishing bioequivalence of inhaled products. Clin Ther. 2009;31:370–85.
Asmus MJ, Liang J, Coowanitwong I, Hochhaus G. In vitro performance characteristics of valved holding chamber and spacer devices with a fluticasone metered-dose inhaler. Pharmacotherapy. 2004;24:159–66.
Woodcock A, Acerbi D, Poli G. Modulite® technology: pharmacodynamic and pharmacokinetic implications. Respir Med. 2002;96:S9–15.
Harrison LI, Soria I, Cline AC, Ekholm BP. Pharmacokinetic differences between chlorofluorocarbon and chlorofluorocarbon-free metered dose inhalers of beclomethasone dipropionate in adult asthmatics. J Pharm Pharmacol. 1999;51:1235–40.
Borgstrom L. In vitro, ex vivo, in vivo veritas. Allergy. 1999;54:88–92.
Newman SP, Chan HK. In vitro/in vivo comparisons in pulmonary drug delivery. J Aerosol Med Pulm Drug Deliv. 2008;21(1):77–84.
Allen DB, Bielory L, Derendorf H, Dluhy R, Colice GL, Szefler SJ. Inhaled corticosteroids: past lessons and future issues. J Allergy Clin Immunol. 2003;112:S1–40.
Borgstrom L, Nilsson M. A method for determination of absolute pulmonary bioavailability of inhaled drugs: terbutaline. Pharm Res. 1990;7:1068–70.
Brown JS, Gordon T, Price O, Asgharian B. Thoracic and respirable particle definitions for human health risk assessment. Part Fibre Toxicol. 2013;10:12.
Goyal N, Hochhaus G. Demonstrating bioequivalence using pharmacokinetics: theoretical considerations across drug classes. Respir Drug Deliv. 2010;1:261–72.
Usmani OS. Exploring aerosol absorption in humans: pharmacokinetics of monodisperse fluticasone propionate. Respir Drug Deliv. 2014;1:155–62.
Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am J Respir Crit Care Med. 2005;172:1497–504.
Newman SP, Wilding IR. Gamma scintigraphy: an in vivo technique for assessing the equivalence of inhaled products. Int J Pharmacol. 1998;170:1–9.
Borgstrom L, Olsson B. Aspects of pharmacokinetic study design to differentiate between different orally inhaled drug products. Respir Drug Deliv. 2010;1:285–92.
Pauwels R, Newman S, Borgstrom L. Airway deposition and airway effects of antiasthma drugs delivered from metered-dose inhalers. Eur Respir J. 1997;10:2127–38.
Gerrity TR, Lee PS, Hass FJ, Marinelli A, Werner P, Lourenco RV. Calculated deposition of inhaled particles in the airway generations of normal subjects. J Appl Physiol. 1979;47:867–73.
Ebina M, Yaegashi H, Chiba R, Takahashi T, Motomiya M, Tanemura M. Hyperreactive site in the airway tree of asthmatic patients revealed by thickening of bronchial muscles: a morphometric study. Am Rev Respir Dis. 1990;141:1327–32.
Ruffin RE, Montgomery JM, Newhouse MT. Site of beta-adrenergic receptors in the respiratory tract: use of fenoterol administered by two methods. Chest. 1978;74:256–60.
Ruffin RE, Dolovich MB, Oldenburg FA, Newhouse MT. The preferential deposition of inhaled isoprotenerol and propanolol in asthmatic patients. Chest. 1981;80:904–6.
Newman SP, Moren F, Trofast E, Talaee N, Clarke SW. Deposition and clinical efficacy of terbutaline sulphate from Turbuhaler, a new multi- dose powder inhaler. Eur Respir J. 1989;2:247–52.
Newman SP, Talaee N, Clarke SW. Salbutamol aerosol delivery in man with the Rondo spacer. Acta Ther. 1991;17:49–58.
Newman SP, Weisz AW, Talaee N, Clarke SW. Improvement of drug delivery with a breath actuated pressurised aerosol for patients with poor inhaler technique. Thorax. 1991;46:712–6.
Hultquist C, Wollmer P, Eklundh G, Jonson B. Effect of inhaled terbutaline sulphate in relation to its deposition in the lungs. Pulm Pharmacol. 1992;5:127–32.
Melchor R, Biddiscombe MF, Mak VH, Short MD, Spiro SG. Lung deposition patterns of directly labelled salbutamol in normal subjects and in patients with reversible airflow obstruction. Thorax. 1993;48:506–11.
Newman SP, Clarke SW. Bronchodilator delivery from Gentlehaler, a new low-velocity pressurized aerosol inhaler. Chest. 1993;103:1442–6.
Vidgren M, Arppe J, Vidgren P, Hyvarinen L, Vainio P, Silvasti M, et al. Pulmonary deposition and clinical response of 99mTc-labelled salbutamol delivered from a novel multiple dose powder inhaler. Pharm Res. 1994;11:1320–4.
Conner D, Davit B. Bioequivalence and drug product assessment in vivo. In: Shargel L, Kanfer I, editors. Generic drug product development: solid oral dosage forms. New York: Marcel Dekker Inc.; 2005. p. 227–78.
Kempsford R, Handel M, Mehta R, De Silva M, Daley-Yates P. Comparison of the systemic pharmacodynamic effects and pharmacokinetics of salmeterol delivered by CFC propellant and non-CFC propellant metered dose inhalers in healthy subjects. Respir Med. 2005;99:S11–9.
Burgess C, Ayson M, Rajasingham S, Crane J, Cioppa GD, Till MD. The extrapulmonary effects of increasing doses of formoterol in patients with asthma. Eur J Clin Pharmacol. 1998;54:141–7.
Kunka R, Andrews S, Pimazzoni M, Callejas S, Ziviani L, Squassante L, et al. Dose proportionality of fluticasone propionate from hydrofluoroalkane pressurized metered dose inhalers (pMDIs) and comparability with chlorofluorocarbon pMDIs. Respir Med. 2000;94:S10–6.
Mollmann H, Wagner M, Meibohm B, Hochhaus G, Barth J, Stockmann R, et al. Pharmacokinetic and pharmacodynamic evaluation of fluticasone propionate after inhaled administration. Eur J Clin Pharmacol. 1998;53:459–67.
GSK Clinical Study Register. The determination of the dose–response characteristics of single inhaled doses (12.5 mcg, 25 mcg, 50 mcg, and 100 mcg) of GR33343G in patients with asthma. a randomised, double-blind comparison with inhaled salbutamol [study ID SLGH04]. http://www.gsk-clinicalstudyregister.com/study/SLGH04#rs. Accessed 20 Mar 2016.
Dahl R, Creemers JP, Noord J, Sips A, Cioppae GD, Thomsone M, et al. Comparable efficacy and tolerability of formoterol (Foradil®) administered via a novel multi-dose dry powder inhaler (Certihaler™) or the Aerolizer® dry powder inhaler in patients with persistent asthma. Respiration. 2004;71:126–33.
Centre for Drug Evaluation and Research. Application number 022383Orig1s000/indacaterol. Medical review. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022383Orig1s000MedR.pdf. Accessed 20 March 2016.
Centre for Drug Evaluation and Research. Application number 204275Orig1s000/fluticasone furoate and vilanterol. Medical review. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204275Orig1s000MedR.pdf. Accessed 20 Mar 2016.
Pearlman DS, Noonan MJ, Tashkin DP, Goldstein MF, Hamedani AG, Kellerman DJ, et al. Comparative efficacy and safety of twice daily fluticasone propionate powder versus placebo in the treatment of moderate asthma. Ann Allergy Asthma Immunol. 1997;78:356–62.
Sheffer AL, LaForce C, Chervinsky P, Pearlman D, Schaberg A. Fluticasone propionate aerosol: efficacy in patients with mild to moderate asthma. Fluticasone Propionate Asthma Study Group. J Fam Pract. 1996;42:369–75.
Centre for Drug Evaluation and Research. Application number 21-949/pulmicort Flexhaler. Medical review (budesonide). http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021949_budesonide_toc.cfm. Accessed 20 March 2016.
Silkoff PE, McClean P, Spino M, Erlich L, Slutsky AS, Zamel N. Dose response relationship and reproducibility of the fall in exhaled nitric oxide after inhaled beclomethasone dipropionate therapy in asthma patients. Chest. 2001;119:1322–8.
Langdon CG, Adler M, Mehra S, Alexander M, Drollmann A. Once-daily ciclesonide 80 or 320 mcg for 12 weeks is safe and effective in patients with persistent asthma. Respir Med. 2005;99:1275–85.
Pearlman DS, Berger WE, Kerwin E, LaForce C, Kundu S, Banerji D. Once-daily ciclesonide improves lung function and is well tolerated by patients with mild-to-moderate persistent asthma. J Allergy Clin Immunol. 2005;116:1206–12.
Centre for Drug Evaluation and Research. Approval package for application number NDA 21-067/Asmanex® Twisthaler. Medical review (mometasone). http://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021067s000_Asmanex_Twisthaler_MedR.pdf. Accessed 20 March 2016.
Bateman ED, Bleecker ER, Lötvall J, Woodcock A, Forth R, Medley H, et al. Dose effect of once-daily fluticasone furoate in persistent asthma: a randomized trial. Respir Med. 2012;106:642–50.
Allen A. The relationship between fluticasone furoate systemic exposure and cortisol suppression. Clin Pharmacokinet. 2013;52:885–96.
Mackie AE, Bye A. The relationship between systemic exposure to fluticasone propionate and cortisol reduction in healthy male volunteers. Clin Pharmacokinet. 2000;39(Suppl 1):47–54.
Grahnen A, Jansson B, Brundin RM, Ling-Andersson A, Lonnebo A, Johansson M, et al. A dose–response study comparing suppression of plasma cortisol induced by fluticasone propionate from Diskhaler and budesonide from Turbuhaler. Eur J Clin Pharmacol. 1997;52:261–7.
Wilson AM, Clark DJ, McFarlane L, Lipworth BJ. Adrenal suppression with high doses of inhaled fluticasone propionate and triamcinolone acetonide in healthy volunteers. Eur J Clin Pharmacol. 1997;53(1):33–7.
Weda M, Zanen P, de Boer AH, Gjaltema D, Ajaoud A, Barends DM, et al. Equivalence testing of salbutamol dry powder inhalers: in vitro impaction results versus in vivo efficacy. Int J Pharmacol. 2002;249(1–2):247–55.
Weda M, Zanen P, de Boer AH, Barends DM, Frijlink HW. An investigation into the predictive value of cascade impactor results for side effects of inhaled salbutamol. Int J Pharmacol. 2004;287(1–2):79–87.
Usmani OS, Biddiscombe MF, Nightingale JA, Underwood SR, Barnes PJ. Effects of bronchodilator particle size in asthmatic patients using monodisperse aerosols. J Appl Physiol. 2003;95(5):2106–12.
Donnelly R, Williams KM, Baker AB, Badcock C, Day RO, Seale JP. Effects of budesonide and fluticasone on 24-hour plasma cortisol—a dose–response study. Am J Respir Crit Care Med. 1997;156:1746–51.
Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109:410–8.
Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O’Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337(20):1405–11.
Clearie KL, Williamson PA, Meldrum K, Gillen M, Carlsson L, Carlholm M, et al. Pharmacokinetic and pharmacodynamic comparison of hydrofluoroalkane and chlorofluorocarbon formulations of budesonide. Br J Clin Pharmacol. 2011;71:504–13.
Needham MJ, Bainbridge P, Harrison LI, Ratner P, 3M Drug Delivery Systems Group, Sylvana Research Group. Comparative pulmonary function & pharmacokinetics of fluticasone propionate and salmeterol xinafoate in asthmatics using 3M Conix™ and Advair Diskus [poster]. Respiratory Drug Delivery (RDD) Europe 2011; 3–6 May 2011; Berlin.
Busse WW, Brazinsky S, Jacobson K, Stricker W, Schmitt K, Vanden Burgt J, et al. Efficacy response of inhaled beclomethasone dipropionate in asthma is proportional to dose and is improved by formulation with a new propellant. J Allergy Clin Immunol. 1999;104:1215–22.
Boorsma M, Andersson N, Larsson P, Ullman A. Assessment of the relative systemic potency of inhaled fluticasone and budesonide. Eur Respir J. 1996;9:1427–32.
Chandran S, Morde N, Rebello J, Chachad S, Purandare S, Malhotra G, et al. A comparative study on systemic pharmacodynamic effects of two HFA formulations of salmeterol xinafoate, in healthy subjects. Eur Respir J. 2010;36(Suppl. 54):351s.
Daley-Yates PT, Price AC, Sisson JR, Pereira A, Dallow N. Beclomethasone dipropionate: absolute bioavailability, pharmacokinetics and metabolism following intravenous, oral, intranasal and inhaled administration in man. Br J Clin Pharmacol. 2001;51:400–9.
Houghton CM, Langley SJ, Singh SD, Holden J, Monici Preti AP, Acerbi D, et al. Comparison of bronchoprotective and bronchodilator effects of a single dose of formoterol delivered by hydrofluoroalkane and chlorofluorocarbon aerosols and dry powder in a double blind, placebo-controlled, crossover study. Br J Clin Pharmacol. 2004;58:359–66.
Acknowledgements
The authors thank Dr. Paul Dorinsky, Ms. Juliet Robello, Ms. Nazma Morde, Ms. Mayuri Mangle and Dr. Sudipta Ganguli for valuable relevant discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
All authors certify that no funding has been received for the preparation of this manuscript.
Conflict of interest
Dr. Usmani reports having received consulting fees, honorarium, institutional grants and payments for lectures from Boehringer Ingelheim, AstraZeneca, Chiesi, Napp, Mundipharma, Aerocrine, GlaxoSmithKline (GSK), Sandoz, Takeda, Edmond Pharma, Zentiva and Cipla outside of the submitted work. Dr Molimard reports having received consulting fees from Novartis Pharma, GSK and Mundipharma outside of the submitted work. Dr Derom reports that he has received travel grants from Boehringer Ingelheim, GSK and AstraZeneca to attend international congresses, participated in advisory boards by Boehringer Ingelheim, Chiesi, Cipla and Astra Zeneca, for which a fee was given, and received speaker’s fees from Boehringer Ingelheim, GSK, Astra Zeneca and Menarini to give scientific presentations to Belgian general practitioner groupings (all of which are not related to this work). His clinical department received financial support from Boehringer Ingelheim and Novartis to perform clinical studies. Drs Gaur, Gogtay, Singh and Malhotra are full-time employees of Cipla Ltd, Mumbai, India.
Rights and permissions
About this article
Cite this article
Usmani, O.S., Molimard, M., Gaur, V. et al. Scientific Rationale for Determining the Bioequivalence of Inhaled Drugs. Clin Pharmacokinet 56, 1139–1154 (2017). https://doi.org/10.1007/s40262-017-0524-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0524-6