Abstract
Objective
The aim of this study was to evaluate the pharmacokinetics (PK) of trastuzumab emtansine (T-DM1) and relevant analytes in patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer and hepatic impairment.
Methods
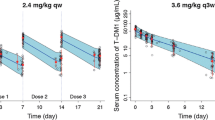
Patients were enrolled in three independent parallel cohorts based on hepatic function per Child–Pugh criteria: normal hepatic function, mild hepatic impairment, and moderate hepatic impairment. Patients received T-DM1 3.6 mg/kg intravenously every 3 weeks. PK samples were collected during cycles 1 and 3, and the PK of T-DM1 and relevant analytes were characterized and compared across cohorts.
Results
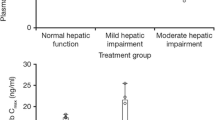
Compared with patients with normal hepatic function (n = 10), T-DM1 clearance at cycle 1 was 1.8- and 4.0-fold faster in the mild (n = 10) and moderate (n = 8) cohorts, respectively. The trend of faster clearance was less apparent in cycle 3, with similar T-DM1 clearance across cohorts (mean ± standard deviation 8.16 ± 3.27 [n = 9], 9.74 ± 3.62 [n = 7], and 8.99 and 10.2 [individual values, n = 2] mL/day/kg for the normal, mild, and moderate cohorts, respectively). T-DM1 clearance at cycle 1 correlated significantly with baseline albumin, aspartate aminotransferase, and HER2 extracellular domain concentrations (p < 0.05). Plasma concentrations of DM1 and DM1-containing catabolites were low and were comparable across cohorts.
Conclusions
No increase in systemic DM1 concentration was observed in patients with mild or moderate hepatic impairment versus those with normal hepatic function. The faster T-DM1 clearance observed at cycle 1 in patients with hepatic impairment appeared to be transient. After repeated dosing (three cycles), T-DM1 exposure in patients with mild and moderate hepatic impairment was within the range seen in those with normal hepatic function.





Similar content being viewed by others
References
Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–90.
Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. 2011;128(2):347–56.
Girish S, Gupta M, Wang B, Lu D, Krop IE, Vogel CL, et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol. 2012;69(5):1229–40.
Shen BQ, Bumbaca D, Saad O, Yue Q, Pastuskovas CV, Khojasteh SC, et al. Catabolic fate and pharmacokinetic characterization of trastuzumab emtansine (T-DM1): an emphasis on preclinical and clinical catabolism. Curr Drug Metab. 2012;13(7):901–10.
Lu D, Girish S, Gao Y, Wang B, Yi JH, Guardino E, et al. Population pharmacokinetics of trastuzumab emtansine (T-DM1), a HER2-targeted antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer: clinical implications of the effect of covariates. Cancer Chemother Pharmacol. 2014;74(2):399–410.
Davis JA, Rock DA, Wienkers LC, Pearson JT. In vitro characterization of the drug-drug interaction potential of catabolites of antibody-maytansinoid conjugates. Drug Metab Dispos. 2012;40(10):1927–34.
Hagemeister FB Jr, Buzdar AU, Luna MA, Blumenschein GR. Causes of death in breast cancer: a clinicopathologic study. Cancer. 1980;46(1):162–7.
O’Reilly SM, Richards MA, Rubens RD. Liver metastases from breast cancer: the relationship between clinical, biochemical and pathological features and survival. Eur J Cancer. 1990;26(5):574–7.
Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for industry. Pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling. 2003. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm072123.pdf. Accessed Aug 2015.
Dere R, Yi JH, Lei C, Saad OM, Huang C, Li Y, et al. PK assays for antibody-drug conjugates: case study with ado-trastuzumab emtansine. Bioanalysis. 2013;5(9):1025–40.
Zhao B, Chen R, O’Connor OA, Gopal AK, Ramchandren R, Goy A, et al. Brentuximab vedotin, an antibody-drug conjugate, in patients with CD30-positive haematologic malignancies and hepatic or renal impairment. Br J Clin Pharmacol. 2016;82(3):696–705.
Garg A, Quartino A, Li J, Jin J, Wada R, Li H, et al. Population pharmacokinetic and covariate analysis of pertuzumab, a HER2-targeted monoclonal antibody, and evaluation of a fixed, non-weight-based dose in patients with a variety of solid tumors. Cancer Chemother Pharmacol. 2014;74:819–29.
Fasanmade AA, Adedokun OJ, Olson A, Strauss R, Davis HM. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmcol Ther. 2010;48(5):297–308.
Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62:779–86.
Kim J, Bronson CL, Hayton WL, Radmacher MD, Roopenian DC, Robinson JM, et al. Albumin turn-over: FcRn-mediated recycling saves as much as albumin from degradation as the liver products. Am J Physiol Gastrointest Liver Physiol. 2006;290:G352–60.
Chih HE, Gikanga B, Yang Y, Zhang B. Identification of amino acid residues responsible for the release of free drug from an antibody-drug conjugate utilizing lysine-succinimidyl ester chemistry. J Pharm Sci. 2011;100:2518–25.
Trastuzumab emtansine: summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002389/WC500158593.pdf. Accessed 14 Sept 2016.
Trastuzumab emtansine: US prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125427lbl.pdf. Accessed 14 Sept 2016.
Acknowledgements
The authors would like to thank the patients and all investigators for their participation in this trial. Patients with mild and moderate hepatic impairment were enrolled by Dr. Jean-Pierre Delort, Dr. Miguel Martin Jimenez, Dr. Joan Albanell Mestres, Dr. Lowell Hart, and Dr. Howard Burris. Please see Online Resource 1 for the full list of study investigators. The authors also thank Mr. Uwe Ziegler, F. Hoffmann-La Roche Ltd, for his support with the statistical analysis. Support for third-party medical writing assistance for this manuscript was provided by F. Hoffmann-La Roche Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Funding
This work was supported by F. Hoffmann-La Roche Ltd.
Conflicts of interest
Chunze Li is an employee of Genentech, Inc. and owns stock in F. Hoffmann-La Roche Ltd and Genentech, Inc. Priya Agarwal, Nataliya Chernyukhin, and Sandhya Girish are employees of and own stock in Genentech, Inc. Ekaterina Gibiansky is a consultant to Genentech, Inc. Jin Yan Jin is an employee of Genentech, Inc. and owns stock in F. Hoffmann-La Roche Ltd and Eli Lilly. Susan Dent received honoraria from F. Hoffmann-La Roche Ltd and Amgen, Inc. Anthony Gonçalves received honoraria/consulting fees, support for travel, and non-financial support from F. Hoffmann-La Roche Ltd. Ihsan Nijem is an employee of Genentech, Inc. and owns stock in F. Hoffmann-La Roche Ltd. Alexander Strasak and Marie-Laurence Harle-Yge are employees of and own stock in F. Hoffmann-La Roche Ltd. Pat LoRusso received honoraria and non-financial support (e.g. provision of writing assistance, medicines, equipment, or administrative support) from, and has served as a consultant to, Genentech, Inc.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, C., Agarwal, P., Gibiansky, E. et al. A Phase I Pharmacokinetic Study of Trastuzumab Emtansine (T-DM1) in Patients with Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer and Normal or Reduced Hepatic Function. Clin Pharmacokinet 56, 1069–1080 (2017). https://doi.org/10.1007/s40262-016-0496-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0496-y