Abstract
Objectives
Our objective was to characterize the population pharmacokinetics of paliperidone after intramuscular administration of its long-acting 3-month formulation palmitate ester at various doses and at different injection sites (deltoid and gluteal muscles).
Methods
This retrospective analysis included pooled data from 651 subjects from one phase I study (single injection of the 3-month formulation) and one phase III study (multiple injections of both 1- and 3-month formulations). A total of 8990 pharmacokinetic samples with valid concentration time points were available for this analysis. Nonlinear mixed-effects modelling of the pooled data was conducted using NONMEM software. Knowledge from a previously developed 1-month formulation model was used as a starting point to build the 3-month formulation model.
Results
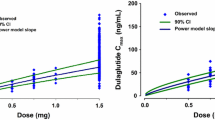
The final model describing the plasma concentrations after administration of the 3-month formulation was a one-compartment model with first-order elimination and two saturable absorption processes (rapid and slow). The apparent volume of distribution estimated for the 3-month formulation was not the same as for the previously modelled 1-month formulation. Apparent clearance (CL), apparent volume of distribution (V), and fraction of the absorbed dose (F3) were estimated to be 3.84 l/h, 1960 L, and 20.9 %. For slow absorption, the maximum absorption rate constant (k a1 max), amount of paliperidone at the absorption site when half of the maximum absorption rate was achieved (k amt1 50), and Hill factor (γ) were estimated to be 90.4 µg/h, 120 mg, and 1.44, respectively. For rapid absorption, the maximum absorption rate constant (k a3 max) and amount of paliperidone at the absorption site when half of the maximum absorption rate was achieved (k amt3 50) were estimated to be 164 µg/h and 21.4 mg, respectively.
Conclusion
The final model with two saturable absorption processes provided a good description of the pharmacokinetic characteristics of paliperidone after intramuscular administration of its long-acting 3-month formulation palmitate ester. In addition to the structural covariates (creatinine clearance on CL, body mass index on V, and injection volume on both absorption rates), injection site and sex were identified as covariates on k a max of the slow absorption process (k a1 max).
Clinical trial registration numbers: NCT01559272, NCT01529515, and NCT01515423






Similar content being viewed by others
References
Keith SJ, Pani L, Nick B, et al. Practical application of pharmacotherapy with long-acting risperidone for patients with schizophrenia. Psychiatr Serv. 2004;55(9):997–1005.
Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216–22.
Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry. 2003;64(11):1308–15.
Valenstein M, Copeland LA, Blow FC, et al. Pharmacy data identify poorly adherent patients with schizophrenia at increased risk for admission. Med Care. 2002;40(8):630–9.
Ascher-Svanum H, Faries DE, Zhu B, et al. Medication adherence and longterm functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006;67(3):453–60.
Remenar JF. Making the leap from daily oral dosing to long-acting injectables: lessons from the antipsychotics. Mol Pharm. 2014;11(6):1739–49.
Janicak PG, Winans EA. Paliperidone ER: a review of the clinical trial data. Neuropsychiatr Dis Treat. 2007;3(6):869–97.
Bhanji NH, Chouinard G, Margolese HC. A review of compliance, depot intramuscular antipsychotics and the new long-acting injectable atypical antipsychotic risperidone in schizophrenia. Eur Neuropsychopharmacol. 2004;14(2):87–92.
Masand PS, Roca M, Turner MS, et al. Partial adherence to antipsychotic medication impacts the course of illness in patients with schizophrenia: a review. Prim Care Companion J Clin Psychiatry. 2009;11(4):147–54.
Weiden PJ, Zygmunt A. The road back: working with the severely mentally ill. Medication noncompliance in schizophrenia. J Pract Psychiatr Behav Health. 1997;3:106–10.
US label of INVEGA® SUSTENNA® (Revised 01/2016). http://www.invegasustenna.com/important-product-information. Accessed 15 Feb 2016.
Samtani MN, Vermeulen A, Stuyckens K. Population pharmacokinetics of intramuscular paliperidone palmitate in patients with schizophrenia: a novel once-monthly, long-acting formulation of an atypical antipsychotic. Clin Pharmacokinet. 2009;48(9):585–600.
Ravenstijn P, Remmerie B, Savitz A, De Meulder M, Hough D, Gopal S, et al. Pharmacokinetics, safety, and tolerability of paliperidone palmitate 3-month formulation in patients with schizophrenia: a phase-1, single-dose, randomized, open-label study. J Clin Pharmacol. 2016;56(3):330–9.
Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830–9.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edn. Arlington: American Psychiatric Association; 2000. (Text revision [DSM-IV-TR]).
De Meulder M, Remmerie BM, de Vries R, et al. Validated LC-MS/Msmethods for the determination of risperidone and the enantiomers of 9-hydroxyrisperidone in human plasma and urine. J Chromatogr. 2008;870(1):8–16.
R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. ISBN 3-900051-07-0. http://www.R-project.org. Accessed 18 Jan 2016.
Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ. NONMEM users guides. Ellicott City: Icon Development Solutions; 1989.
Zhang L, Beal SL, Sheiner LB. Simultaneous vs. sequential analysis for population PK/PD data I: best-case performance. J Pharmacokinet Pharmacodyn. 2003;30(6):387–404.
Gisleskog PO, Karlsson MO, Beal SL. Use of prior information to stabilize a population data analysis. J Pharmacokinet Pharmacodyn. 2002;29(5–6):473–505.
Dansirikul C, Silber HE, Karlsson MO. Approaches to handling pharmacodynamic baseline responses. J Pharmacokinet Pharmacodyn. 2008;35(3):269–83.
Jonsson EN, Karlsson MO. Automated covariate model building within NONMEM. Pharm Res. 1998;15(9):1463–8.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
Hirano K, Yamada H. Studies on the absorption of practically water-insoluble drugs following injection: IV. An approach for predicting relative intramuscular absorption rates of a drug in oily solution, aqueous suspension and aqueous surfactant solution in rats. Chem Pharm Bull. 1981;29(5):1410–5.
Hirano K, Yamada H. Studies on the absorption of practically water-insoluble drugs following injection: VIII. Comparison of the subcutaneous absorption rates from aqueous suspensions in the mouse, rat, and rabbit. J Pharm Sci. 1983;72(6):608–12.
Zuidema J, Kadir F, Titulear HAC, et al. Release and absorption rate of intramuscularly and subcutaneously injected pharmaceuticals (II). Int J Pharm. 1994;105(3):189–207.
Khandelwal A, Harling K, Jonsson EN, Hooker AC, Karlsson MO. A fast method for testing covariates in population PK/PD Models. AAPS J. 2011;13(3):464–72.
Zhou H. Pharmacokinetic strategies in deciphering atypical drug absorption profiles. J Clin Pharmacol. 2003;43(3):211–27.
Hirano K, Ichihashi T, Yamada H. Studies on the absorption of practically water-insoluble drugs following injection: II. Intramuscular absorption from aqueous suspensions in rats. Chem Pharm Bull. 1981;29(3):817–27.
Magnusson MO, Samtani MN, Plan EL, Jonsson EN, Rossenu S, Vermeulen A, et al. Population pharmacokinetic simulations of two paliperidone palmitate formulations. Poster presented at PAGE 24 (2015) Abstr 3643 http://www.page-meeting.org/?abstract=3643.
Label of INVEGA® TRINZA® (Revised 03/2016). https://www.janssenmd.com/pdf/invega-trinza/INVEGA-TRINZA_PI.pdf. Accessed 4 July 2016.
Educational Dose Illustrator for: INVEGA SUSTENNA® (paliperidone palmitate) INVEGA TRINZA™ (paliperidone palmitate) http://www.educationaldoseillustrator.com/pp3m/schizophrenia).
Acknowledgments
The authors thank Tinka Tuinstra PhD, an employee of AUTHOR! et al. BV, for her medical writing assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. O. Magnusson, E. L. Plan and E. N. Jonsson are employees of Pharmetheus and received consultancy fees from Janssen Research & Development. M. N. Samtani, S. Rossenu, A. Vermeulen, and A. Russu are employees of Janssen Research & Development and own Johnson & Johnson stocks and/or stock options.
Additional information
M. O. Magnusson and M. N. Samtani have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Magnusson, M.O., Samtani, M.N., Plan, E.L. et al. Population Pharmacokinetics of a Novel Once-Every 3 Months Intramuscular Formulation of Paliperidone Palmitate in Patients with Schizophrenia. Clin Pharmacokinet 56, 421–433 (2017). https://doi.org/10.1007/s40262-016-0459-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0459-3