Abstract
Dolutegravir (DTG), elvitegravir (EVG) and raltegravir (RAL) are members of the latest class of antiretrovirals (ARVs) that have become available to treat human immunodeficiency virus (HIV) infection: integrase strand transfer inhibitors (INSTIs). INSTIs are potent inhibitors of the HIV integrase enzyme, with protein binding–adjusted concentration inhibiting viral replication by 90/95 % [IC90/95] values in the low nanogram per millilitre range, and they retain antiviral activity against strains of HIV with acquired resistance to other classes of ARVs. Each of the INSTIs has unique pharmacokinetic/pharmacodynamic properties, influencing its role in clinical use in specific subsets of patients. RAL and DTG have minimal drug–drug interaction profiles, as their metabolism has minimal cytochrome P450 (CYP) involvement. Conversely, EVG metabolism occurs primarily via CYP3A4 and requires pharmacokinetic boosting to achieve systemic exposures amenable to once-daily dosing. EVG and DTG have the added benefit of availability of fixed-dose combination tablets, allowing for convenient and simplified ARV regimens. RAL is the only INSTI to be listed as a preferred agent in the current US perinatal treatment guidelines. All three INSTIs are recommended regimens for treatment-naïve individuals in the US adult and adolescent HIV treatment guidelines. This review summarizes and compares the pharmacokinetics and pharmacodynamics of the INSTIs, and describes specific pharmacokinetic considerations for special patient conditions: hepatic impairment, renal dysfunction, pregnancy and co-infections.
Similar content being viewed by others
References
Department of Health and Human Services (DHHS). Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 9 Feb 2016.
Min S, Sloan L, DeJesus E, Hawkins T, McCurdy L, Song I, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. Aids. 2011;25(14):1737–45.
Min S, Song I, Borland J, Chen S, Lou Y, Fujiwara T, et al. Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. Antimicrob Agents Chemother. 2010;54(1):254–8.
Tivicay® [package insert]. Research Triangle Park: Viiv Healthcare, August 2015.
Song I, Borland J, Chen S, Patel P, Wajima T, Peppercorn A, et al. Effect of food on the pharmacokinetics of the integrase inhibitor dolutegravir. Antimicrob Agents Chemother. 2012;56(3):1627–9.
Kobayashi M, Yoshinaga T, Seki T, Wakasa-Morimoto C, Brown KW, Ferris R, et al. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother. 2011;55(2):813–21.
Weller S, Chen S, Borland J, Savina P, Wynne B, Piscitelli SC. Bioequivalence of a dolutegravir, abacavir, and lamivudine fixed-dose combination tablet and the effect of food. J Acquir Immune Defic Syndr. 2014;66(4):393–8.
Reese MJ, Savina PM, Generaux GT, Tracey H, Humphreys JE, Kanaoka E, et al. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos. 2013;41(2):353–61.
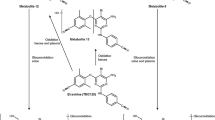
Castellino S, Moss L, Wagner D, Borland J, Song I, Chen S, et al. Metabolism, excretion, and mass balance of the HIV-1 integrase inhibitor dolutegravir in humans. Antimicrob Agents Chemother. 2013;57(8):3536–46.
van Lunzen J, Maggiolo F, Arribas JR, Rakhmanova A, Yeni P, Young B, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis. 2012;12(2):111–8. doi:10.1016/S1473-3099(11)70290-0.
Raffi F, Jaeger H, Quiros-Roldan E, Albrecht H, Belonosova E, Gatell JM, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2013;13(11):927–35. doi:10.1016/S1473-3099(13)70257-3.
Zhang J, Hayes S, Sadler BM, Minto I, Brandt J, Piscitelli S, et al. Population pharmacokinetics of dolutegravir in HIV-infected treatment-naive patients. Br J Clin Pharmacol. 2015;80(3):502–14.
Vitekta® [package insert]. Foster City: Gilead Sciences, Inc., July 2015.
Stribild® [package insert]. Foster City: Gilead Sciences, Inc., July 2015.
Genvoya® [package insert]. Foster City: Gilead Sciences, Inc., November 2015.
Kawaguchi I, Ishikawa T, Ishibashi M, Irie S, Kakee A, editors. Safety and pharmacokinetics of single oral dose of JTK-303/GS-9137, a novel HIV integrase inhibitor, in healthy volunteers. In: 13th conference on retroviruses and opportunistic infections; 2006.
Kearney B, Mathias A, Zhong L, editors. Pharmacokinetics/pharmacodynamics of GS-9137 an HIV integrase inhibitor in treatment-naive and experienced patients. In: International workshop on clinical pharmacology of HIV therapy; 2006.
German P, Warren D, West S, Hui J, Kearney BP. Pharmacokinetics and bioavailability of an integrase and novel pharmacoenhancer-containing single-tablet fixed-dose combination regimen for the treatment of HIV. J Acquir Immune Defic Syndr. 2010;55(3):323–9.
DeJesus E, Berger D, Markowitz M, Cohen C, Hawkins T, Ruane P, et al. Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-naive and treatment-experienced patients. J Acquir Immune Defic Syndr. 2006;43(1):1–5.
German P, Warren D, Wei L, Zhong L, Hui J, Kearney B, editors. Effect of food on pharmacokinetics of elvitegravir, emtricitabine, tenofovir DF and the pharmacoenhancer GS-9350 as a fixed-dose combination tablet. In: 49th Interscience conference on antimicrobial agents and chemotherapy (ICAAC). San Francisco; 2009.
Shiomi M, Matsuki S, Ikeda A, Ishikawa T, Nishino N, Kimura M, et al. Effects of a protein-rich drink or a standard meal on the pharmacokinetics of elvitegravir, cobicistat, emtricitabine and tenofovir in healthy Japanese male subjects: a randomized, three-way crossover study. J Clin Pharmacol. 2014;54(6):640–8.
Patel P, Song I, Borland J, Patel A, Lou Y, Chen S, et al. Pharmacokinetics of the HIV integrase inhibitor S/GSK1349572 co-administered with acid-reducing agents and multivitamins in healthy volunteers. J Antimicrob Chemother. 2011;66(7):1567–72. doi:10.1093/jac/dkr139.
Ramanathan S, Shen G, Hinkle J, Enejosa J, Kearney B, editors. Pharmacokinetic evaluation of drug interactions with ritonavir-boosted HIV integrase inhibitor GS-9137 (elvitegravir) and acid-reducing agents. In: 8th international workshop on clinical pharmacology of HIV therapy; 2007.
Ramanathan S, Wright M, West S, Kearney B, editors. Pharmacokinetics, metabolism, and excretion of ritonavir-boosted GS-9137 (elvitegravir). In: 8th international workshop on clinical pharmacology of HIV therapy; 2007.
Isentress® [package insert]. Whitehouse Station: Merck & Co., Inc.; February 2015.
Siccardi M, D’Avolio A, Rodriguez-Novoa S, Cuenca L, Simiele M, Baietto L, et al. Intrapatient and interpatient pharmacokinetic variability of raltegravir in the clinical setting. Ther Drug Monit. 2012;34(2):232–5.
Barau C, Furlan V, Yazdanpanah Y, Fagard C, Molina J-M, Taburet A-M, et al. Characterization of binding of raltegravir to plasma proteins. Antimicrob Agents Chemother. 2013;57(10):5147–50.
Kiser JJ, Bumpass JB, Meditz AL, Anderson PL, Bushman L, Ray M, et al. Effect of antacids on the pharmacokinetics of raltegravir in human immunodeficiency virus-seronegative volunteers. Antimicrob Agents Chemother. 2010;54(12):4999–5003.
Arab-Alameddine M, Fayet-Mello A, Lubomirov R, Neely M, di Iulio J, Owen A, et al. Population pharmacokinetic analysis and pharmacogenetics of raltegravir in HIV-positive and healthy individuals. Antimicrob Agents Chemother. 2012;56(6):2959–66.
Brainard DM, Friedman EJ, Jin B, Breidinger SA, Tillan MD, Wenning LA, et al. Effect of low-, moderate-, and high-fat meals on raltegravir pharmacokinetics. J Clin Pharmacol. 2011;51(3):422–7.
Iwamoto M, Wenning L, Petry A, Laethem M, De Smet M, Kost J, et al. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin Pharmacol Ther. 2008;83(2):293–9.
Markowitz M, Morales-Ramirez JO, Nguyen B-Y, Kovacs CM, Steigbigel RT, Cooper DA, et al. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2006;43(5):509–15.
Petry A, Wenning L, Laethem M, De Smet M, Kost J, Merschman S et al., editors. Safety, tolerability, and pharmacokinetics after single and multiple doses of MK-0518 in healthy subjects. In: 46th interscience conference on antimicrobial agents and chemotherapy; 2006.
Kassahun K, McIntosh I, Cui D, Hreniuk D, Merschman S, Lasseter K, et al. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the human immunodeficiency virus 1 integrase enzyme. Drug Metab Dispos. 2007;35(9):1657–63.
Wenning L, Petry A, Kost J, Jin B, Breidinger S, DeLepeleire I, et al. Pharmacokinetics of raltegravir in individuals with UGT1A1 polymorphisms. Clin Pharmacol Ther. 2009;85(6):623–7.
Eron JJ Jr, Rockstroh JK, Reynes J, Andrade-Villanueva J, Ramalho-Madruga JV, Bekker LG, et al. Raltegravir once daily or twice daily in previously untreated patients with HIV-1: a randomised, active-controlled, phase 3 non-inferiority trial. Lancet Infect Dis. 2011;11(12):907–15. doi:10.1016/S1473-3099(11)70196-7.
Rizk ML, Hang Y, Luo W-L, Su J, Zhao J, Campbell H, et al. Pharmacokinetics and pharmacodynamics of once-daily versus twice-daily raltegravir in treatment-naive HIV-infected patients. Antimicrob Agents Chemother. 2012;56(6):3101–6.
Debinski HS, Lee CS, Danks JA, Mackenzie PI, Desmond PV. Localization of uridine 5′-diphosphate-glucuronosyltransferase in human liver injury. Gastroenterology. 1995;108(5):1464–9.
Furlan V, Demirdjian S, Bourdon O, Magdalou J, Taburet AM. Glucuronidation of drugs by hepatic microsomes derived from healthy and cirrhotic human livers. J Pharmacol Exp Ther. 1999;289(2):1169–75.
George J, Murray M, Byth K, Farrell GC. Differential alterations of cytochrome P450 proteins in livers from patients with severe chronic liver disease. Hepatology (Baltimore, Md). 1995;21(1):120–8.
US Food and Drug Administration. Pharmacokinetics in patients with impaired hepatic function: study design, data analysis and impact on dosing and labeling. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm072123.pdf. Accessed 5 March 2016.
US Food and Drug Administration. Dolutegravir. 2013. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204790Orig1s000ClinPharmR.pdf. Accessed 18 Feb 2016.
Song IH, Borland J, Savina PM, Chen S, Patel P, Wajima T, et al. Pharmacokinetics of single-dose dolutegravir in HIV-seronegative subjects with moderate hepatic impairment compared to healthy matched controls. Clin Pharmacol Drug Dev. 2013;2(4):342–8. doi:10.1002/cpdd.55.
US Food and Drug Administration. Elvitegravir. 2011. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203100Orig1s000ClinPharmR.pdf. Accessed 18 Feb 2016.
Custodio JM, Rhee M, Shen G, Ling KH, Kearney BP, Ramanathan S. Pharmacokinetics and safety of boosted elvitegravir in subjects with hepatic impairment. Antimicrob Agents Chemother. 2014;58(5):2564–9. doi:10.1128/aac.02180-13.
Iwamoto M, Hanley WD, Petry AS, Friedman EJ, Kost JT, Breidinger SA, et al. Lack of a clinically important effect of moderate hepatic insufficiency and severe renal insufficiency on raltegravir pharmacokinetics. Antimicrob Agents Chemother. 2009;53(5):1747–52. doi:10.1128/aac.01194-08.
Calza L, Danese I, Colangeli V, Manfredi R, Magistrelli E, Verucchi G, et al. Plasma concentrations of efavirenz, darunavir/ritonavir and raltegravir in HIV-HCV-coinfected patients without liver cirrhosis in comparison with HIV-monoinfected patients. Infect Dis (London, England). 2015;47(9):625–36. doi:10.3109/23744235.2015.1034169.
Hernandez-Novoa B, Moreno A, Perez-Elias MJ, Quereda C, Dronda F, Casado JL, et al. Raltegravir pharmacokinetics in HIV/HCV-coinfected patients with advanced liver cirrhosis (Child-Pugh C). J Antimicrob Chemother. 2014;69(2):471–5. doi:10.1093/jac/dkt386.
Barau C, Braun J, Vincent C, Haim-Boukobza S, Molina JM, Miailhes P, et al. Pharmacokinetic study of raltegravir in HIV-infected patients with end-stage liver disease: the LIVERAL-ANRS 148 study. Clin Infect Dis. 2014;59(8):1177–84. doi:10.1093/cid/ciu515.
US Food and Drug Administration. Pharmacokinetics in patients with impaired renal function: study design, data analysis and impact on dosing and labeling. March 2010. http://www.fda.gov/downloads/Drugs/…/Guidances/UCM204959.pdf. Accessed 7 March 2016.
Weller S, Borland J, Chen S, Johnson M, Savina P, Wynne B, et al. Pharmacokinetics of dolutegravir in HIV-seronegative subjects with severe renal impairment. Eur J Clin Pharmacol. 2014;70(1):29–35. doi:10.1007/s00228-013-1590-9.
Pozniak A, Arribas JR, Gathe J, Gupta SK, Post FA, Bloch M, et al. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48 week results from a single-arm, multi-center, open-label, phase 3 study. J Acquir Immune Defic Syndr. 2015;. doi:10.1097/qai.0000000000000908.
Molto J, Sanz-Moreno J, Valle M, Cedeno S, Bonal J, Bouarich H, et al. Minimal removal of raltegravir by hemodialysis in HIV-infected patients with end-stage renal disease. Antimicrob Agents Chemother. 2010;54(7):3047–8. doi:10.1128/AAC.00363-10.
Giguere P, la Porte C, Zhang G, Cameron B. Pharmacokinetics of darunavir, etravirine and raltegravir in an HIV-infected patient on haemodialysis. Aids. 2009;23(6):740–2. doi:10.1097/QAD.0b013e328328f79d.
Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed 8 March 2016.
Colbers A, Greupink R, Burger D. Pharmacological considerations on the use of antiretrovirals in pregnancy. Curr Opin Infect Dis. 2013;26(6):575–88. doi:10.1097/QCO.0000000000000017.
Jeong H, Choi S, Song JW, Chen H, Fischer JH. Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica. 2008;38(1):62–75. doi:10.1080/00498250701744633.
McCormack SA, Best BM. Protecting the fetus against HIV infection: a systematic review of placental transfer of antiretrovirals. Clin Pharmacokinet. 2014;53(11):989–1004. doi:10.1007/s40262-014-0185-7.
Mulligan N, Best BM, Capparelli E, Stek A, Barr E, Smith E, Chakhtoura N, Wang J, Burchett S, Mirochnick M. Dolutegravir pharmacokinetics in HIV-infected pregnant and postpartum women. CROI 2016. Boston; February 22–25, 2016. Abstract #438.
Schalkwijk S, Colbers A, Konopnicki D, Greupink R, Russel FG, Burger D, et al. First reported use of elvitegravir and cobicistat during pregnancy. AIDS. 2016;30(5):807–8. doi:10.1097/QAD.0000000000000976.
DeJesus E, Rockstroh JK, Henry K, Molina JM, Gathe J, Ramanathan S, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012;379(9835):2429–38. doi:10.1016/S0140-6736(12)60918-0.
Watts DH, Stek A, Best BM, Wang J, Capparelli EV, Cressey TR, et al. Raltegravir pharmacokinetics during pregnancy. J Acquir Immune Defic Syndr. 2014;67(4):375–81. doi:10.1097/qai.0000000000000318.
Blonk MI, Colbers AP, Hidalgo-Tenorio C, Kabeya K, Weizsacker K, Haberl AE, et al. Raltegravir in HIV-1-infected pregnant women: pharmacokinetics, safety, and efficacy. Clin Infect Dis. 2015;61(5):809–16. doi:10.1093/cid/civ366.
Rizk ML, Hang Y, Luo WL, Su J, Zhao J, Campbell H, et al. Pharmacokinetics and pharmacodynamics of once-daily versus twice-daily raltegravir in treatment-naive HIV-infected patients. Antimicrob Agents Chemother. 2012;56(6):3101–6. doi:10.1128/AAC.06417-11.
Grinsztejn B, De Castro N, Arnold V, Veloso VG, Morgado M, Pilotto JH, et al. Raltegravir for the treatment of patients co-infected with HIV and tuberculosis (ANRS 12 180 REFLATE TB): a multicentre, phase 2, non-comparative, open-label, randomised trial. Lancet Infect Dis. 2014;14(6):459–67.
Burger DM, Magis-Escurra C, van den Berk GE, Gelinck LB. Pharmacokinetics of double-dose raltegravir in two patients with HIV infection and tuberculosis. AIDS. 2010;24(2):328–30.
Min S, Sloan L, DeJesus E, Hawkins T, McCurdy L, Song I, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS. 2011;25(14):1737–45. doi:10.1097/QAD.0b013e32834a1dd9.
Clotet B, Feinberg J, van Lunzen J, Khuong-Josses MA, Antinori A, Dumitru I, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383(9936):2222–31. doi:10.1016/S0140-6736(14)60084-2.
Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893):700–8. doi:10.1016/S0140-6736(13)61221-0.
Castagna A, Maggiolo F, Penco G, Wright D, Mills A, Grossberg R, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis. 2014;210(3):354–62. doi:10.1093/infdis/jiu051.
DeJesus E, Berger D, Markowitz M, Cohen C, Hawkins T, Ruane P, et al. Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-naive and treatment-experienced patients. J Acquir Immune Defic Syndr. 2006;43(1):1–5. doi:10.1097/01.qai.0000233308.82860.2f.
Zolopa AR, Berger DS, Lampiris H, Zhong L, Chuck SL, Enejosa JV, et al. Activity of elvitegravir, a once-daily integrase inhibitor, against resistant HIV type 1: results of a phase 2, randomized, controlled, dose-ranging clinical trial. J Infect Dis. 2010;201(6):814–22. doi:10.1086/650698.
Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379(9835):2439–48. doi:10.1016/S0140-6736(12)60917-9.
US Food and Drug Administration. Raltegravir. 2007. Part 1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022145_ClinPharmR_P1.pdf. Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022145_ClinPharmR_P2.pdf. Accessed 18 Feb 2016.
Steigbigel RT, Cooper DA, Kumar PN, Eron JE, Schechter M, Markowitz M, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359(4):339–54. doi:10.1056/NEJMoa0708975.
Markowitz M, Morales-Ramirez JO, Nguyen BY, Kovacs CM, Steigbigel RT, Cooper DA, et al. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2006;43(5):509–15. doi:10.1097/QAI.0b013e31802b4956.
Grinsztejn B, Nguyen BY, Katlama C, Gatell JM, Lazzarin A, Vittecoq D, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007;369(9569):1261–9. doi:10.1016/S0140-6736(07)60597-2.
Fletcher CV. Drug interactions should be evaluated in patients. Clin Pharmacol Ther. 2010;88(5):585–7. doi:10.1038/clpt.2010.213.
Fletcher CV. Editorial commentary: cerebrospinal fluid inhibitory quotients of antiretroviral drugs. Clin Infect Dis. 2015;60(2):318–20. doi:10.1093/cid/ciu775.
US Food and Drug Administration. Guidance for industry, antiviral product development, conducting and submitting virology studies to the agency. 2006. http://www.fda.gov/OHRMS/DOCKETS/98fr/05d-0183-gdl0002-01.pdf. Accessed 18 Feb 2016.
Lennox JL, DeJesus E, Lazzarin A, Pollard RB, Madruga JV, Berger DS, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374(9692):796–806. doi:10.1016/S0140-6736(09)60918-1.
Walmsley SL, Antela A, Clumeck N, Duiculescu D, Eberhard A, Gutierrez F, et al. Dolutegravir plus abacavir–lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–18. doi:10.1056/NEJMoa1215541.
Raffi F, Rachlis A, Stellbrink HJ, Hardy WD, Torti C, Orkin C, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735–43. doi:10.1016/S0140-6736(12)61853-4.
Simoni JM, Nance RM, Delaney JAC, Wilson I, Aunon F, Safren SA, et al. HIV viral load in US clinics over time: trends and predictions from CNICS. Conference on retroviruses and opportunistic infections (CROI). February 22, 2016; Boston.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors acknowledge support from the following grants from the National Institutes of Health: 1R01HD085887-01A1 (to Kimberly K. Scarsi) and RO1 AI124965-01 and UM1AI06701 (to Courtney V. Fletcher).
Conflict of interest
Anthony T. Podany, Kimberly K. Scarsi and Courtney V. Fletcher have no conflicts of interest that are directly relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Podany, A.T., Scarsi, K.K. & Fletcher, C.V. Comparative Clinical Pharmacokinetics and Pharmacodynamics of HIV-1 Integrase Strand Transfer Inhibitors. Clin Pharmacokinet 56, 25–40 (2017). https://doi.org/10.1007/s40262-016-0424-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0424-1