Abstract
Background and Objective
We recently published analyses regarding the predictive performance of physiologically based pharmacokinetic (PBPK) models, submitted to the US Food and Drug Administration (FDA), for the effect of cytochrome P450 (CYP) inhibitors on the pharmacokinetics of substrate drugs. We now analyze and summarize the predictive performance of PBPK models for the effect of CYP3A inducers on a substrate’s pharmacokinetics.
Methods
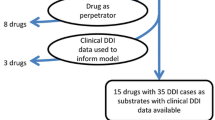
This analysis was based on 11 substrate PBPK models, developed by six sponsors, using a commercial PBPK software, with 13 clinical interaction studies. Four CYP3A inducers were used: rifampicin, rifabutin, carbamazepine, and efavirenz. Sponsors either directly used the software-provided inducer models or verified these models’ induction magnitude prior to use. The metric for assessing predictive performance was the R predicted/observed value [R predicted/observed = (predicted mean exposure ratio)/(observed mean exposure ratio)], with the exposure ratio defined as maximum plasma concentration (C max) or area under the plasma concentration–time curve (AUC) with and without an inducer.
Results
In 77 % (10/13; AUCR) and 83 % (10/12; C max R) of the cases, the R predicted/observed values were within 1.25-fold of the observed data. Cases with R predicted/observed values >1.25-fold (>twofold for all three AUCR) were under-predictions as a result of using the PBPK software’s default rifampicin model. Improved predictions were observed when the rifampicin model was modified by increasing the induction potency.
Conclusion
Based on submissions to the FDA, and similar to our previous findings for CYP inhibition, we observed good agreement between PBPK-predicted and observed effect of CYP3A inducers on substrate pharmacokinetics. Verification of the inducer model appears to be crucial for improved predictive performance.



Similar content being viewed by others
References
Zhang L, Zhang YD, Zhao P, Huang SM. Predicting drug-drug interactions: an FDA perspective. AAPS J. 2009;11:300–6.
Wagner C, Pan Y, Hsu V, Grillo JA, Zhang L, Reynolds KS, et al. Predicting the effect of cytochrome P450 inhibitors on substrate drugs: analysis of physiologically based pharmacokinetic modeling submissions to the US Food and Drug Administration. Clin Pharmacokinet. 2015;54:117–27.
Vieira MD, Kim MJ, Apparaju S, Sinha V, Zineh I, Huang SM, et al. PBPK Model describes the effects of comedication and genetic polymorphism on systemic exposure of drugs that undergo multiple clearance pathways. Clin Pharmacol Ther. 2014;95:550–7.
Einolf HJ, Chen L, Fahmi OA, Gibson CR, Obach RS, Shebley M, et al. Evaluation of various static and dynamic modeling methods to predict clinical CYP3A induction using in vitro CYP3A4 mRNA induction data. Clin Pharmacol Ther. 2014;95:179–88.
Guest EJ, Aarons L, Houston JB, Rostami-Hodjegan A, Galetin A. Critique of the twofold measure of prediction success for ratios: application for the assessment of drug–drug interactions. Drug Metab Dispos. 2011;39:170–3.
Xu Y, Zhou Y, Hayashi M, Shou M, Skiles GL. Simulation of clinical drug-drug interactions from hepatocyte CYP3A4 induction data and its potential utility in trial designs. Drug Metab Dispos. 2011;39:1139–48.
Baneyx G, Parrott N, Meille C, Iliadis A, Lave T. Physiologically based pharmacokinetic modeling of CYP3A4 induction by rifampicin in human: influence of time between substrate and inducer administration. Eur J Pharm Sci. 2014;56:1–15.
Acknowledgments
The authors acknowledge constructive input from Dr. Shiew Mei Huang. CW was supported by an appointment to the ORISE Research Participation Program at the Center for Drug Evaluation and Research (CDER) administered by the Oak Ridge Institute for Science and Education through an agreement between the US Department of Energy and CDER.
A part of this work was presented at the American Society for Clinical Pharmacology and Therapeutics (ASCPT) Annual Meeting, March 3–7, 2015, New Orleans, LA, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
The contents of this manuscript do not reflect the views or policies of the US Food and Drug Administration (FDA) or its staff. No official support or endorsement by the FDA is intended or should be inferred.
Rights and permissions
About this article
Cite this article
Wagner, C., Pan, Y., Hsu, V. et al. Predicting the Effect of CYP3A Inducers on the Pharmacokinetics of Substrate Drugs Using Physiologically Based Pharmacokinetic (PBPK) Modeling: An Analysis of PBPK Submissions to the US FDA. Clin Pharmacokinet 55, 475–483 (2016). https://doi.org/10.1007/s40262-015-0330-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-015-0330-y