Abstract
Background
Meropenem is an anti-Gram-negative antimicrobial, the time-dependent activity of which may be maximized through administration by continuous infusion.
Objectives
The objectives of this study were to characterize the pharmacokinetics of continuous infusion meropenem in relation to body size and Cockcroft–Gault estimated creatinine clearance (CLCR) in overweight and obese patients with stable and unstable kidney function with the intent of creating a nomogram for optimal dosing.
Patients and Methods
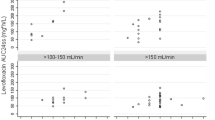
Patients from a single institution with a body mass index ≥25 kg/m2 receiving meropenem by continuous infusion with measurement of meropenem steady-state concentrations (C ss) were identified. Individual Bayesian estimates of meropenem volume of distribution of the central compartment (V c) and clearance (CL) were calculated and relationships to body size descriptors and CLCR estimated using these body size descriptors were defined by regression. Kidney function stability was defined based on median absolute deviation, stratification by the ratio of maximum to minimum serum creatinine (SCr) and individual patient-level regression of SCr over time. The influence of kidney function stability on meropenem CL estimation by CLCR was tested.
Results
A total of 375 patients (77.9 % male) with 846 C ss values (62.4 % of patients with ≥2 measurements) were identified. The median daily dose of meropenem and frequency of infusion bag changes were 2000 mg/day and four times per day, respectively. The meropenem C ss values were ≥16, ≥8, ≥4, and ≥2 mg/L for 41.1, 76.1, 97.4, and 99.9 % of observations, respectively. The median (range) age, weight, and BMI were 66 (24–90) years, 90 (70–250) kg, and 30.8 (25.1–81.6) kg/m2, respectively. The mean [standard deviation (SD)] serum creatinine at baseline was 1.57 (1.37) mg/dL. The mean (SD) V c was 28.1 (1.36) L and not related to body size, while CL was 8.85 (6.40) L/h and best related to CLCR estimated using adjusted body weight (ABW). The meropenem CL to CLCR relationship was not significantly impacted by the presence or absence of kidney function stability. The user-friendly dosing nomogram based on CLCR estimated using ABW showed that optimal drug exposure [Css ≥ minimum inhibitory concentration (MIC)] may be obtained even against multi-drug resistant (MDR) pathogens when considering dosages up to 1250 mg every 6 h by continuous infusion.
Conclusions
Meropenem CL is best estimated using CLCR with ABW in patients with a BMI ≥25 kg/m2 and this relationship is not altered by unstable kidney function. Application of our dosing nomogram may improve the care of overweight and obese patients with severe MDR Gram-negative infections treated with meropenem by continuous infusion.



Similar content being viewed by others
References
Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81.
Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71–87.
Wickman C, Kramer H. Obesity and kidney disease: potential mechanisms. Semin Nephrol. 2013;33(1):14–22.
Hites M, Taccone FS, Wolff F, et al. Broad-spectrum beta-lactams in obese non-critically ill patients. Nutr Diabetes. 2014;4:e119.
Pai MP. Estimating the glomerular filtration rate in obese adult patients for drug dosing. Adv Chronic Kidney Dis. 2010;17(5):e53–62.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
Pea F, Viale P, Cojutti P, et al. Dosing nomograms for attaining optimum concentrations of meropenem by continuous infusion in critically ill patients with severe gram-negative infections: a pharmacokinetics/pharmacodynamics-based approach. Antimicrob Agents Chemother. 2012;56(12):6343–8.
MERREM® IV (meropenem for injection) [product label]. Wilmington: Astra Zeneca Pharmaceuticals LP. 2006. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050706s022lbl.pdf. Accessed 13 Dec 2014.
Martinez MN, Papich MG, Drusano GL. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob Agents Chemother. 2012;56(6):2795–805.
Krueger WA, Bulitta J, Kinzig-Schippers M, et al. Evaluation by monte carlo simulation of the pharmacokinetics of two doses of meropenem administered intermittently or as a continuous infusion in healthy volunteers. Antimicrob Agents Chemother. 2005;49(5):1881–9.
Zavascki AP, Carvalhaes CG, Picao RC, et al. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther. 2010;8(1):71–93.
Cohen J. Confronting the threat of multidrug-resistant Gram-negative bacteria in critically ill patients. J Antimicrob Chemother. 2013;68(3):490–1.
Franceschi L, Cojutti P, Baraldo M, et al. Stability of generic meropenem solutions for administration by continuous infusion at normal and elevated temperatures. Ther Drug Monit. 2014;36(5):674–6.
Manning L, Wright C, Ingram PR, et al. Continuous infusions of meropenem in ambulatory care: clinical efficacy, safety and stability. PLoS One. 2014;9(7):e102023.
Carlier M, Stove V, Verstraete AG, et al. Stability of generic brands of meropenem reconstituted in isotonic saline. Minerva Anestesiol. 2015;81(3):283–7.
Udy AA, Roberts JA, Boots RJ, et al. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet. 2010;49(1):1–16.
Li C, Du X, Kuti JL, et al. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother. 2007;51(5):1725–30.
Tam VH, Nikolaou M. A novel approach to pharmacodynamic assessment of antimicrobial agents: new insights to dosing regimen design. PLoS Comput Biol. 2011;7(1):e1001043.
Tam VH, Schilling AN, Neshat S, et al. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49(12):4920–7.
Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098.
Pai MP, Paloucek FP. The origin of the “ideal” body weight equations. Ann Pharmacother. 2000;34(9):1066–9.
Bauer LA, Edwards WA, Dellinger EP, et al. Influence of weight on aminoglycoside pharmacokinetics in normal weight and morbidly obese patients. Eur J Clin Pharmacol. 1983;24(5):643–7.
Janmahasatian S, Duffull SB, Ash S, et al. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44(10):1051–65.
National Heart, Lung, and Blood Institute. Classification of overweight and obesity by BMI, waist circumference, and associated disease risks. https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmi_dis.htm. Accessed 13 Mar 2015.
Cheatham SC, Fleming MR, Healy DP, et al. Steady-state pharmacokinetics and pharmacodynamics of meropenem in morbidly obese patients hospitalized in an intensive care unit. J Clin Pharmacol. 2014;54(3):324–30.
Roberts JA, Ulldemolins M, Roberts MS, et al. Therapeutic drug monitoring of beta-lactams in critically ill patients: proof of concept. Int J Antimicrob Agents. 2010;36(4):332–9.
Kays MB, Fleming MR, Cheatham SC, et al. Comparative pharmacokinetics and pharmacodynamics of doripenem and meropenem in obese patients. Ann Pharmacother. 2014;48(2):178–86.
Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect. 2011;17(8):1135–41.
Pea F, Cojutti P, Merelli M, et al. Treatment of consecutive episodes of multidrug-resistant bacterial pleurisy with different aetiology in a heart transplant candidate: proof of concept of pharmacokinetic/pharmacodynamic optimisation of antimicrobial therapy at the infection site. Int J Antimicrob Agents. 2014;44(6):570–1.
Hsu AJ, Tamma PD. Treatment of multidrug-resistant Gram-negative infections in children. Clin Infect Dis. 2014;58(10):1439–48.
Acknowledgments
No external funding was used in the conduct of this study. Federico Pea has received funds for speaking at symposia organized on behalf of Astra Zeneca. Manjunath Pai and Piergiorgio Cojutti have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pai, M.P., Cojutti, P. & Pea, F. Pharmacokinetics and Pharmacodynamics of Continuous Infusion Meropenem in Overweight, Obese, and Morbidly Obese Patients with Stable and Unstable Kidney Function: A Step Toward Dose Optimization for the Treatment of Severe Gram-Negative Bacterial Infections. Clin Pharmacokinet 54, 933–941 (2015). https://doi.org/10.1007/s40262-015-0266-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-015-0266-2