Abstract
Background and objectives
A fixed-dose combination of the bronchodilators umeclidinium and vilanterol is in development for the long-term, once-daily treatment of chronic obstructive pulmonary disease (COPD). We characterized the pharmacokinetics of umeclidinium and vilanterol in ≈1,635 patients with COPD, evaluating the impact of patient demographics and baseline characteristics on umeclidinium and vilanterol exposure.
Methods
Plasma concentrations of umeclidinium and vilanterol were evaluated in patients enrolled in two phase III, randomized, double-blind, parallel-group, placebo-controlled trials using inhaled umeclidinium/vilanterol combination therapy and inhaled umeclidinium and vilanterol monotherapies as treatments. Population-pharmacokinetic models were developed using non-linear mixed-effects analyses, performed using NONMEM® software. A likelihood-based approach was used to characterize the data below limit of quantification. Umeclidinium and vilanterol exposures at clinical doses were simulated based on the population model.
Results
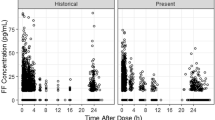
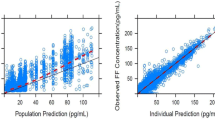
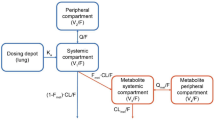
For the umeclidinium and vilanterol population-pharmacokinetic analyses, 1,635 and 1,637 patients provided 8,498 and 8,405 observations, respectively. Umeclidinium and vilanterol pharmacokinetics were best described by a two-compartment model with first-order absorption. For umeclidinium, bodyweight, age, and creatinine clearance (CLCR) were statistically significant covariates for apparent inhaled clearance (CL/F); bodyweight was a statistically significant covariate for volume of distribution of central compartment (V 2/F).The population parameter estimates namely CL/F and V 2/F for umeclidinium were 218 L/h and 1,160 L and 40.9 L/h and 268 L for vilanterol.
For vilanterol, bodyweight and age were statistically significant covariates for CL/F. The effect of covariates on umeclidinium and vilanterol systemic exposure was marginal. The population model indicates that a 10 % increase in bodyweight will result in a 2 % increase in CL/F for umeclidinium and vilanterol and 6 % increase in umeclidinium V 2/F. A 10 % increase in age will provide a 7 and 4 % decrease in umeclidinium and vilanterol CL/F, respectively. A 10 % decrease in CLCR will result in a 3 % decrease in umeclidinium CL/F. Umeclidinium and vilanterol population-pharmacokinetic model-based systemic exposure predictions showed no pharmacokinetic interactions between umeclidinium and vilanterol when administered in combination.
Conclusions
There were no apparent pharmacokinetic interactions when umeclidinium and vilanterol were co-administered in patients with COPD. The effects of patient demographics, including age, bodyweight, and CLCR, on umeclidinium or vilanterol systemic exposure were minimal, and therefore no dose adjustments are necessary.





Similar content being viewed by others
References
Celli BR, MacNee W. ATS ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46.
Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2011. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdf. Accessed 28 Jan 2014.
World Health Organisation. Chronic obstructive pulmonary disease (COPD): burden of COPD. 2011. http://www.who.int/respiratory/copd/burden/en/index.html. Accessed 28 Jan 2014.
Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538–46.
Feldman G, Walker RR, Brooks J, et al. Safety and tolerability of the GSK573719/vilanterol combination in patients with COPD. Am J Respir Crit Care Med. 2012;185:A2938.
Celli B, Crater, G, Kilbride, S, et al. Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest. 2014; in press.
Hankinson JL, Kawut SM, Shahar E, et al. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the Multi-Ethnic Study of Atherosclerosis (MESA) lung study. Chest. 2010;137:138–45.
Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87.
Manali ED, Lyberopoulos P, Triantafillidou C, et al. MRC Chronic Dyspnea Scale: relationships with cardiopulmonary exercise testing and 6-minute walk test in idiopathic pulmonary fibrosis patients: a prospective study. BMC Pulm Med. 2010;10:32.
European Medicines Agency. Guideline on reporting the results of population pharmacokinetic analyses. 2007.
Food and Drug Administration. Guidance for industry: population pharmacokinetics. 1999.
Ahn JE, Karlsson MO, Dunne A, et al. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn. 2008;35:401–21.
Holford N. The visual predictive check: superiority to standard diagnostic (Rorschach) plots. Presented at the Population Approach Group in Europe (PAGE), Pamplona, Spain. 2005; Abstract 738.
Zevin S, Benowitz NL. Drug interactions with tobacco smoking: an update. Clin Pharmacokinet. 1999;36:425–38.
Bieth B. Population pharmacokinetics of QVA149, the fixed dose combination of indacaterol maleate and glycopyrronium bromide in chronic obstructive pulmonary disease (COPD) patients. Presented at the Population Approach Group in Europe (PAGE), Glasgow Scotland. 2013; Abstract 2800.
Connolly MJ, Crowley JJ, Charan NB, et al. Impaired bronchodilator response to albuterol in healthy elderly men and women. Chest. 1995;108:401–6.
Rowe JW, Andres R, Tobin JD, et al. The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol. 1976;31:155–63.
Kelleher D, Hardes K, et al. Effect of severe renal impairment (SRI) on umeclidinium (UMEC) and vilanterol (VI) pharmacokinetics (PK). Eur Respir J. 2013;42(Suppl. 57):881s.
Mehta R, Hardes K, Kelleher D, et al. Effect of moderate hepatic impairment (MHI) on umeclidinium (UMEC) and vilanterol (VI) pharmacokinetics (PK). Eur Respir J. 2013;42(Suppl. 57):751s.
Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28:481–504.
Bergstrand M, Karlsson MO. Handling data below the limit of quantification in mixed effect models. AAPS J. 2009;11:371–80.
Acknowledgments
Editorial support was provided by Joanne Parker of Fishawack Indicia Ltd, funded by GlaxoSmithKline.
Ethical standards
All patients were required to give written, informed consent. The studies were approved by local review boards/ethics committees and were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Financial disclosures
This study was funded by GlaxoSmithKline.
Conflict of interest
All authors are employees of GlaxoSmithKline and own stock/stock options in GlaxoSmithKline.
Author information
Authors and Affiliations
Corresponding author
Additional information
ClinicalTrials.gov identifiers NCT01313637; NCT01313650.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goyal, N., Beerahee, M., Kalberg, C. et al. Population Pharmacokinetics of Inhaled Umeclidinium and Vilanterol in Patients with Chronic Obstructive Pulmonary Disease. Clin Pharmacokinet 53, 637–648 (2014). https://doi.org/10.1007/s40262-014-0143-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-014-0143-4