Abstract
Background and Objective
Linifanib is a multi-targeted receptor tyrosine kinase inhibitor, which can inhibit members of the vascular endothelial growth factor and platelet-derived growth factor receptor families. The objective of this analysis was to characterize the population pharmacokinetics of linifanib in cancer patients.
Methods
We pooled 7,351 linifanib plasma concentrations from 1,010 cancer patients enrolled in 13 clinical studies. Population pharmacokinetic modelling was performed using NONMEM version 7.2. The covariates that were screened included the cancer type, co-medications, creatinine clearance, formulation, fed status, liver function markers (bilirubin, blood urea nitrogen [BUN], aspartate aminotransferase [AST], alanine aminotransferase [ALT]), albumin, age, sex, race, body weight, surface area and body mass index.
Results
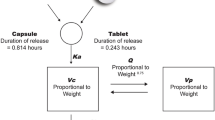
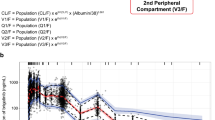
A two-compartment model with first-order absorption and disposition best described linifanib pharmacokinetics. An increase in body weight was associated with less than proportional increases in volumes of distribution. Subjects with hepatocellular carcinoma and renal cell carcinoma were estimated to have 63 and 86 % larger volumes of distribution, respectively, than subjects with the other cancer types. Females had 25 % slower oral clearance (CL/F) than males, while subjects with colorectal cancer had 41 % faster CL/F than other subjects. For linifanib bioavailability, subjects with refractory acute myeloid leukaemia or myelodysplastic syndrome had 43 % lower bioavailability, evening doses were associated with 27 % lower bioavailability than morning doses, and administration of linifanib under fed conditions decreased the bioavailability by 14 %. Finally, the oral solution formulation showed two-fold faster absorption than the tablet formulations.
Conclusion
The use of mixed-effects modelling allowed robust assessment of the impact of the concomitant effects of body size, different cancer types, formulation, diurnal variation, sex and food on linifanib pharmacokinetics. The developed population pharmacokinetic model describes linifanib concentrations adequately and can be used to conduct simulations or to evaluate the linifanib exposure–response relationship.





Similar content being viewed by others
References
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57.
Zetter P, Bruce R. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49(1):407–24.
Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368–80.
Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103(2):159.
Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–60.
Perona R. Cell signalling: growth factors and tyrosine kinase receptors. Clin Transl Oncol. 2006;8(2):77–82.
Homsi J, Daud AI. Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors. Cancer Control. 2007;14(3):285.
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42.
Reck M, Von Pawel J, Zatloukal Pv, et al. Overall survival with cisplatin–gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol. 2010;21(9):1804–9.
Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50.
Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18(2):338–40.
Dai Y, Hartandi K, Ji Z, et al. Discovery of N-(4-(3-amino-1 H-indazol-4-yl) phenyl)-N′-(2-fluoro-5-methylphenyl) urea (ABT-869), a 3-aminoindazole-based orally active multitargeted receptor tyrosine kinase inhibitor. J Med Chem. 2007;50(7):1584–97.
Albert DH, Tapang P, Magoc TJ, et al. Preclinical activity of ABT-869, a multitargeted receptor tyrosine kinase inhibitor. Mol Cancer Ther. 2006;5(4):995–1006.
Linifanib. Drugs R D. 2010;10(2):111–22.
Toh HC, Chen PJ, Carr BI, et al. Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer. 2013;119(2):380–7.
Asahina H, Tamura Y, Nokihara H, et al. An open-label, phase 1 study evaluating safety, tolerability, and pharmacokinetics of linifanib (ABT-869) in Japanese patients with solid tumors. Cancer Chemother Pharmacol. 2012;69(6):1477–86.
Wong CI, Koh TS, Soo R, et al. Phase I and biomarker study of ABT-869, a multiple receptor tyrosine kinase inhibitor, in patients with refractory solid malignancies. J Clin Oncol. 2009;27(28):4718–26.
Steinberg J, Tan E, Wei-Peng Y. Preliminary analysis of ABT-869 safety, pharmacokinetics and efficacy in three phase 2 solid tumor studies [abstract no. 477P]. 33rd Congress of the European Society for Medical Oncology; Stockholm; 12–16 Sep 2008.
Humerickhouse R, Gupta N, Goh B. Phase 1 comparison of pharmacokientics, safety and efficacy with low versus high doses of ABT-869 in refractory solid tumors [abstract no. 476P]. 33rd Congress of the European Society for Medical Oncology; Stockholm; 12–16 Sep 2008.
Wang ES, Yee K, Koh LP, et al. Phase 1 trial of linifanib (ABT-869) in patients with refractory or relapsed acute myeloid leukemia. Leuk Lymphoma. 2012;53(8):1543–51.
Gupta N, Yan Z, LoRusso P, Ricker J, Carlson D, Pradhan R. Assessment of the effect of food on the oral bioavailability and assessment of diurnal variation in the pharmacokinetics of linifanib. Mol Cancer Ther. 2009;8(12 Suppl):B53.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
Tan EH, Goss GD, Salgia R, et al. Phase 2 trial of linifanib (ABT-869) in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2011;6(8):1418–25.
Tannir NM, Wong YN, Kollmannsberger CK, et al. Phase 2 trial of linifanib (ABT-869) in patients with advanced renal cell cancer after sunitinib failure. Eur J Cancer. 2011;47(18):2706–14.
Etienne-Grimaldi M, Cardot J, François E, et al. Chronopharmacokinetics of oral tegafur and uracil in colorectal cancer patients. Clin Pharmacol Ther. 2007;83(3):413–5.
Hassan M, Öberg G, Bekassy A, et al. Pharmacokinetics of high-dose busulphan in relation to age and chronopharmacology. Cancer Chemother Pharmacol. 1991;28(2):130–4.
Koren G, Langevin AM, Olivieri N, Giesbrecht E, Zipursky A, Greenberg M. Diurnal variation in the pharmacokinetics and myelotoxicity of mercaptopurine in children with acute lymphocytic leukemia. Arch Pediatr Adolesc Med. 1990;144(10):1135.
Muggia FM, Wu X, Spicer D, et al. Phase I and pharmacokinetic study of oral UFT, a combination of the 5-fluorouracil prodrug tegafur and uracil. Clin Cancer Res. 1996;2(9):1461–7.
Vassal G, Challine D, Koscielny S, et al. Chronopharmacology of high-dose busulfan in children. Cancer Res. 1993;53(7):1534–7.
Metzger G, Massari C, Etienne M-C, et al. Spontaneous or imposed circadian changes in plasma concentrations of 5-fluorouracil coadministered with folinic acid and oxaliplatin: relationship with mucosal toxicity in patients with cancer. Clin Pharmacol Ther. 1994;56(2):190–201.
Petit E, Milano G, Lévi F, Thyss A, Bailleul F, Schneider M. Circadian rhythm-varying plasma concentration of 5-fluorouracil during a five-day continuous venous infusion at a constant rate in cancer patients. Cancer Res. 1988;48(6):1676–9.
Squalli A, Oustrin J, Houin G. Clinical chronopharmacokinetics of doxorubicin (DXR). Annu Rev Chronopharmacol. 1989;5:393–6.
Lévi F. Circadian rhythms in 5-fluorouracil pharmacology and therapeutic applications. In: Rustum YM. Fluoropyrimidines in cancer therapy. Totowa: Humana Press, 2003. p. 107–28.
Lévi F, Okyar A, Dulong S, Innominato PF, Clairambault J. Circadian timing in cancer treatments. Annu Rev Pharmacol Toxicol. 2010;50:377–421.
Zhang C, Denti P, Decloedt E, et al. Model-based approach to dose optimization of lopinavir/ritonavir when co-administered with rifampicin. Br J Clin Pharmacol. 2012;73(5):758–67.
Iwahori T, Takeuchi H, Matsuno N, et al. Pharmacokinetic differences between morning and evening administration of cyclosporine and tacrolimus therapy. Transplant Proc. 2005;37:1739–40.
Lemmer B. Chronopharmacokinetics: implications for drug treatment. J Pharm Pharmacol. 1999;51(8):887–90.
Belanger P. Chronobiological variation in the hepatic elimination of drugs and toxic chemical agents. Annu Rev Chronopharmacol. 1988;4:1–46.
Radzialowski FM, Bousquet WF. Daily rhythmic variation in hepatic drug metabolism in the rat and mouse. J Pharmacol Exp Ther. 1968;163(1):229–38.
Gachon F, Olela FF, Schaad O, Descombes P, Schibler U. The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab. 2006;4(1):25–36.
Lu J-F, Eppler SM, Wolf J, et al. Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin Pharmacol Ther. 2006;80(2):136–45.
Li J, Karlsson MO, Brahmer J, et al. CYP3A phenotyping approach to predict systemic exposure to EGFR tyrosine kinase inhibitors. J Natl Cancer Inst. 2006;98(23):1714–23.
Bow EJ, Kilpartick MG, Scott BA, Clinich JJ, Cheang MS. Acute myeloid leukemia in Manitoba. Cancer. 1994;74(1):52–60.
Bow EJ, Loewen R, Cheang MS, Shore TB, Rubinger M, Schacter B. Cytotoxic therapy-induced d-xylose malabsorption and invasive infection during remission-induction therapy for acute myeloid leukemia in adults. J Clin Oncol. 1997;15(6):2254–61.
Johnson E, MacGowan A, Potter M, et al. Reduced absorption of oral ciprofloxacin after chemotherapy for haematological malignancy. J Antimicrob Chemother. 1990;25(5):837–42.
Sitar DS, Aoki FY, Bow EJ. Acyclovir bioavailability in patients with acute myelogenous leukemia treated with daunorubicin and cytarabine. J Clin Pharmacol. 2008;48(8):995–8.
Bow E, Meddings J. Intestinal mucosal dysfunction and infection during remission-induction therapy for acute myeloid leukaemia. Leukemia. 2006;20(12):2087–92.
Klotz U. Pathophysiological and disease-induced changes in drug distribution volume: pharmacokinetic implications. Clin Pharmacokinet. 1976;1(3):204–18.
Gupta N, Chiu Y, Toh H, et al. Preliminary pharmacokinetics and safety comparison of Child–Pugh A (CPA) vs Child–Pugh B (CPB) patients enrolled in a phase 2 study in hepatocellular carcinoma (HCC). Presented at Annals of Oncology; 2010.
Frohna P, Lu J, Eppler S, et al. Evaluation of the absolute oral bioavailability and bioequivalence of erlotinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in a randomized, crossover study in healthy subjects. J Clin Pharmacol. 2006;46(3):282–90.
Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523.
Conflicts of Interest
This study was sponsored by AbbVie Inc. AbbVie Inc. contributed to the study design; research; data interpretation; and writing, review and approval of the manuscript for publication. Ahmed Hamed Salem, Denise Koenig and Dawn Carlson are employees of AbbVie Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salem, A.H., Koenig, D. & Carlson, D. Pooled Population Pharmacokinetic Analysis of Phase I, II and III Studies of Linifanib in Cancer Patients. Clin Pharmacokinet 53, 347–359 (2014). https://doi.org/10.1007/s40262-013-0121-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-013-0121-2