Abstract
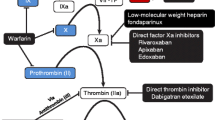
New oral anticoagulants (OACs) that directly inhibit Factor Xa (FXa) or thrombin have been developed for the long-term prevention of thromboembolic disorders. These novel agents provide numerous benefits over older vitamin K antagonists (VKAs) due to major pharmacological differences. VKAs are economical and very well characterized, but have important limitations that can outweigh these advantages, such as slow onset of action, narrow therapeutic window and unpredictable anticoagulant effect. VKA-associated dietary precautions, monitoring and dosing adjustments to maintain international normalized ratio (INR) within therapeutic range, and bridging therapy, are inconvenient for patients, expensive, and may result in inappropriate use of VKA therapy. This may lead to increased bleeding risk or reduced anticoagulation and increased risk of thrombotic events. The new OACs have rapid onset of action, low potential for food and drug interactions, and predictable anticoagulant effect that removes the need for routine monitoring. FXa inhibitors, e.g. rivaroxaban and apixaban, are potent, oral direct inhibitors of prothrombinase-bound, clot-associated or free FXa. Both agents have a rapid onset of action, a wide therapeutic window, little or no interaction with food and other drugs, minimal inter-patient variability, and display similar pharmacokinetics in different patient populations. Since both are substrates, co-administration of rivaroxaban and apixaban with strong cytochrome P450 (CYP) 3A4 and permeability glycoprotein (P-gp) inhibitors and inducers can result in substantial changes in plasma concentrations due to altered clearance rates; consequently, their concomitant use is contraindicated and caution is required when used concomitantly with strong CYP3A4 and P-gp inducers. Although parenteral oral direct thrombin inhibitors (DTIs), such as argatroban and bivalirudin, have been on the market for years, DTIs such as dabigatran are novel synthetic thrombin antagonists. Dabigatran etexilate is a low-molecular-weight non-active pro-drug that is administered orally and converted rapidly to its active form, dabigatran—a potent, competitive and reversible DTI. Dabigatran has an advantage over the indirect thrombin inhibitors, unfractionated heparin and low-molecular-weight heparin, in that it inhibits free and fibrin-bound thrombin. The reversible binding of dabigatran may provide safer and more predictable anticoagulant treatment than seen with irreversible, non-covalent thrombin inhibitors, e.g. hirudin. Dabigatran shows a very low potential for drug–drug interactions. However, co-administration of dabigatran etexilate with other anticoagulants and antiplatelet agents can increase the bleeding risk. Although the new agents are pharmacologically better than VKAs—particularly in terms of fixed dosing, rapid onset of action, no INR monitoring and lower risk of drug interactions—there are some differences between them: the bioavailability of dabigatran is lower than rivaroxaban and apixaban, and so the dabigatran dosage required is higher; lower protein binding of dabigatran reduces the variability related to albuminaemia. The risk of metabolic drug–drug interactions also appears to differ between OACs: VKAs > rivaroxaban > apixaban > dabigatran. The convenience of the new OACs has translated into improvements in efficacy and safety as shown in phase III randomized trials. The new anticoagulants so far offer the greatest promise and opportunity for the replacement of VKAs.




Similar content being viewed by others
References
Almquist HJ, Mecchi E, Klose AA. Estimation of the antihaemorrhagic vitamin. Biochem J. 1938;32(11):1897–903.
Dam H, Schonheyder F. The occurrence and chemical nature of vitamin K. Biochem J. 1936;30(5):897–901.
Dam H, Schonheyder F, Tage-Hansen E. Studies on the mode of action of vitamin K. Biochem J. 1936;30(6):1075–9.
Link KP. The discovery of dicumarol and its sequels. Circulation. 1959;19(1):97–107.
Mann FD, Mann JD, Bollman JL. The coagulation defect of vitamin K deficiency compared with that caused by dicumarol. J Lab Clin Med. 1950;36(2):234–7.
Stehle S, Kirchheiner J, Lazar A, et al. Pharmacogenetics of oral anticoagulants: a basis for dose individualization. Clin Pharmacokinet. 2008;47(9):565–94.
Rost S, Fregin A, Koch D, et al. Compound heterozygous mutations in the gamma-glutamyl carboxylase gene cause combined deficiency of all vitamin K-dependent blood coagulation factors. Br J Haematol. 2004;126(4):546–9.
Sadler JE. Medicine: K is for koagulation. Nature. 2004;427(6974):493–4.
Breckenridge A, Orme ML. The plasma half lives and the pharmacological effect of the enantiomers of warfarin in rats. Life Sci. 1972;11(7):337–45.
Jahnchen E, Meinertz T, Gilfrich HJ, et al. The enantiomers of phenprocoumon: pharmacodynamic and pharmacokinetic studies. Clin Pharmacol Ther. 1976;20(3):342–9.
Meinertz T, Kasper W, Kahl C, et al. Anticoagulant activity of the enantiomers of acenocoumarol. Br J Clin Pharmacol. 1978;5(2):187–8.
Schmidt W, Jahnchen E. Stereoselective drug distribution and anticoagulant potency of the enantiomers of phenprocoumon in rats. J Pharm Pharmacol. 1977;29(5):266–71.
Ufer M. Comparative pharmacokinetics of vitamin K antagonists: warfarin, phenprocoumon and acenocoumarol. Clin Pharmacokinet. 2005;44(12):1227–46.
Thijssen HH, Drittij MJ, Vervoort LM, et al. Altered pharmacokinetics of R- and S-acenocoumarol in a subject heterozygous for CYP2C9*3. Clin Pharmacol Ther. 2001;70(3):292–8.
de Vries JX, Volker U. Determination of the plasma protein binding of the coumarin anticoagulants phenprocoumon and its metabolites, warfarin and acenocoumarol, by ultrafiltration and high-performance liquid chromatography. J Chromatogr. 1990;529(2):479–85.
Harder S, Thurmann P. Clinically important drug interactions with anticoagulants: an update. Clin Pharmacokinet. 1996;30(6):416–44.
Greenblatt DJ, von Moltke LL. Interaction of warfarin with drugs, natural substances, and foods. J Clin Pharmacol. 2005;45(2):127–32.
Hillman MA, Wilke RA, Caldwell MD, et al. Relative impact of covariates in prescribing warfarin according to CYP2C9 genotype. Pharmacogenetics. 2004;14(8):539–47.
Khan T, Wynne H, Wood P, et al. Dietary vitamin K influences intra-individual variability in anticoagulant response to warfarin. Br J Haematol. 2004;124(3):348–54.
Miao L, Yang J, Huang C, et al. Contribution of age, body weight, and CYP2C9 and VKORC1 genotype to the anticoagulant response to warfarin: proposal for a new dosing regimen in Chinese patients. Eur J Clin Pharmacol. 2007;63(12):1135–41.
Momary KM, Shapiro NL, Viana MA, et al. Factors influencing warfarin dose requirements in African-Americans. Pharmacogenomics. 2007;8(11):1535–44.
Eriksson BI, Quinlan DJ, Eikelboom JW. Novel oral factor Xa and thrombin inhibitors in the management of thromboembolism. Ann Rev Med. 2011;62:41–57.
Keeling D, Baglin T, Tait C, et al. Guidelines on oral anticoagulation with warfarin: fourth edition. Br J Haematol. 2011;154(3):311–24.
Bungard TJ, Ghali WA, Teo KK, et al. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160(1):41–6.
White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med. 2007;167(3):239–45.
Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet. 2009;48(1):1–22.
Laux V, Perzborn E, Kubitza D, et al. Preclinical and clinical characteristics of rivaroxaban: a novel, oral, direct factor Xa inhibitor. Semin Thromb Hemost. 2007;33(5):515–23.
Roehrig S, Straub A, Pohlmann J, et al. Discovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3- [4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene-2-carboxamide (BAY 59-7939): an oral, direct factor Xa inhibitor. J Med Chem. 2005;48(19):5900–8.
Perzborn E, Strassburger J, Wilmen A, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct Factor Xa inhibitor. J Thromb Haemost. 2005;3(3):514–21.
Perzborn E, Kubitza D, Misselwitz F. Rivaroxaban. A novel, oral, direct factor Xa inhibitor in clinical development for the prevention and treatment of thromboembolic disorders. Hamostaseologie. 2007;27(4):282–9.
Kubitza D, Becka M, Mueck W, et al. Rivaroxaban (BAY 59-7939)—an oral, direct Factor Xa inhibitor—has no clinically relevant interaction with naproxen. Br J Clin Pharmacol. 2007;63(4):469–76.
Kubitza D, Becka M, Mueck W, et al. Safety, tolerability, pharmacodynamics, and pharmacokinetics of rivaroxaban—an oral, direct factor Xa inhibitor—are not affected by aspirin. J Clin Pharmacol. 2006;46(9):981–90.
Kubitza D, Becka M, Mueck W, et al. Co-administration of rivaroxaban—a novel, oral, direct Factor Xa inhibitor—and clopidogrel in healthy subjects [abstract no. P1272]. Eur Heart J. 2007;28(Suppl 1):189.
Kubitza D, Becka M, Roth A, et al. Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin. 2008;24(10):2757–65.
Kubitza D, Becka M, Wensing G, et al. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct Factor Xa inhibitor—after multiple dosing in healthy male subjects. Eur J Clin Pharm. 2005;61(12):873–80.
Kubitza D, Becka M, Zuehlsdorf M, et al. Effect of food, an antacid, and the H2 antagonist ranitidine on the absorption of BAY 59-7939 (rivaroxaban), an oral, direct factor Xa inhibitor, in healthy subjects. J Clin Pharmacol. 2006;46(5):549–58.
Kubitza D, Becka M, Zuehlsdorf M, et al. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59-7939) in healthy subjects. J Clin Pharmacol. 2007;47(2):218–26.
Mueck W, Borris LC, Dahl OE, et al. Population pharmacokinetics and pharmacodynamics of once- and twice-daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost. 2008;100(3):453–61.
Mueck W, Lensing AW, Agnelli G, et al. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet. 2011;50(10):675–86.
Bayer Pharma AG. Rivaroxaban summary of product characteristics. 2008. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf. Accessed 1 Dec 2012.
Kubitza D, Becka M, Voith B, et al. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78(4):412–21.
Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol. 2010;70(5):703–12.
Lang D, Freudenberger C, Weinz C. In vitro metabolism of rivaroxaban, an oral, direct factor Xa inhibitor, in liver microsomes and hepatocytes of rats, dogs, and humans. Drug Metab Dispos. 2009;37(5):1046–55.
Gross PL, Weitz JI. New anticoagulants for treatment of venous thromboembolism. Arterioscler Thromb Vasc Biol. 2008;28(3):380–6.
Kubitza D, Becka M, Mueck W, et al. The effect of extreme age, and gender on the pharmacology and tolerability of rivaroxaban: an oral, direct factor Xa inhibitor. Blood. 2006;108(11):271–2.
Halabi A, Kubitza D, Zuehlsdorf M, et al. Effect of hepatic impairment on the pharmacokinetics, pharmacodynamics and tolerability of rivaroxaban: an oral, direct factor Xa inhibitor [abstract no. P-M-635]. J Thromb Haemost 2007 5(Suppl 2).
Gnoth MJ, Buetehorn U, Muenster U, et al. In vitro and in vivo P-glycoprotein transport characteristics of rivaroxaban. J Pharmacol Exp Ther. 2011;338(1):372–80.
Mueck W, Becka M, Kubitza D, et al. Population model of the pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor xa inhibitor—in healthy subjects. Int J Clin Pharmacol Ther. 2007;45(6):335–44.
Mueck W, Eriksson BI, Bauer KA, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in patients undergoing major orthopaedic surgery. Clin Pharmacokinet. 2008;47(3):203–16.
Agnelli G, Gallus A, Goldhaber SZ, et al. Treatment of proximal deep-vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59-7939): the ODIXa-DVT (Oral Direct Factor Xa Inhibitor BAY 59-7939 in patients with acute symptomatic deep-vein thrombosis) study. Circulation. 2007;116(2):180–7.
Wong PC, Pinto DJ, Zhang D. Preclinical discovery of apixaban, a direct and orally bioavailable factor Xa inhibitor. J Thromb Thrombolysis. 2011;31(4):478–92.
Pinto DJ, Orwat MJ, Koch S, et al. Discovery of 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide (apixaban, BMS-562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem. 2007;50(22):5339–56.
Wong PC, Jiang X. Apixaban, a direct factor Xa inhibitor, inhibits tissue-factor induced human platelet aggregation in vitro: comparison with direct inhibitors of factor VIIa, XIa and thrombin. Thromb Haemost. 2010;104(2):302–10.
He K, Luettgen JM, Zhang D, et al. Preclinical pharmacokinetics and pharmacodynamics of apixaban, a potent and selective factor Xa inhibitor. Eur J Drug Metab Pharm. 2011;36(3):129–39.
Wong PC, Crain EJ, Xin B, et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost. 2008;6(5):820–9.
Barrett YC, Wang Z, Frost C, et al. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost. 2010;104(6):1263–71.
Wong PC, Watson CA, Crain EJ. Arterial antithrombotic and bleeding time effects of apixaban, a direct factor Xa inhibitor, in combination with antiplatelet therapy in rabbits. J Thromb Haemost. 2008;6(10):1736–41.
Wong P, Watson C, Knabb R, Crain E. The combination of apixaban, a direct factor Xa inhibitor, with heparin or enoxaparin in rabbits elicits additive antithrombotic effects, with low bleeding [abstract no. 933]. Annual Congress of the European Society of Cardiology (ESC) Munich; 30 Aug–3 Sep 2008.
Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37(1):74–81.
Frost CE, Nepal S, Barrett Y, et al. Effects of age and gender on the single-dose pharmacokinetics (PK) and pharmacodynamics (PD) of apixaban [abstract no. PP-MO-407]. J Thromb Haemost. 2009;7(Suppl 2):455.
Prom R, Spinler SA. The role of apixaban for venous and arterial thromboembolic disease. Ann Pharmacother. 2011;45(10):1262–83.
Song Y, Cui Y, Li T, et al. Apixaban pharmacokinetics and pharmacodynamics in healthy Chinese subjects [abstract no. 22]. J Clin Pharmacol. 2010;50:1062.
Upreti VV, Wang J, Barrett YC, et al. Effect of body weight on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor, in healthy subjects [abstract no. 16]. J Clin Pharmacol. 2010;50:1060.
BMS/Pfizer. Eliquis (apixaban) summary of product characteristics. 2011. http://www.eliquis.com/PDF/ELIQUIS%20%C2%AE%20(apixaban)%20SmPC.pdf. Accessed 1 Dec 2012.
Wang L, Zhang D, Raghavan N, et al. In vitro assessment of metabolic drug-drug interaction potential of apixaban through cytochrome P450 phenotyping, inhibition, and induction studies. Drug Metab Dispos. 2010;38(3):448–58.
Frost C, Wang J, Nepal S, et al. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor [abstract no. 139]. J Clin Pharmacol. 2009;49:1123.
Vakkalagadda B, Frost C, Wang J, et al. Effect of rifampin on the pharmacokinetics of apixaban, an oral direct inhibitor of factor Xa [abstract no. 143]. J Clin Pharmacol. 2009;49:1124.
European Medicines Agency Press Office. AstraZeneca withdraws its application for Ximelagatran 36-mg film-coated tablets. 2006. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2010/02/WC500074073.pdf. Accessed 1 Dec 2012.
Eisert WG, Hauel N, Stangier J, et al. Dabigatran: an oral novel potent reversible nonpeptide inhibitor of thrombin. Arterioscler Thromb Vasc Biol. 2010;30(10):1885–9.
Hankey GJ, Eikelboom JW. Dabigatran etexilate: a new oral thrombin inhibitor. Circulation. 2011;123(13):1436–50.
Huntington JA, Baglin TP. Targeting thrombin: rational drug design from natural mechanisms. Trends Pharmacol Sci. 2003;24(11):589–95.
van Ryn J, Hauel N, Waldman L, et al. Dabigatran inhibits both clot-bound and fluid-phase thrombin in vitro: comparison to heparin and hirudin [abstract no. 570]. Arterioscler Thromb Vasc Biol. 2008;28:e136–7.
Weitz JI, Hudoba M, Massel D, et al. Clot-bound thrombin is protected from inhibition by heparin-antithrombin III but is susceptible to inactivation by antithrombin III-independent inhibitors. J Clin Invest. 1990;86(2):385–91.
Maegdefessel L, Linde T, Krapiec F, et al. In vitro comparison of dabigatran, unfractionated heparin, and low-molecular-weight heparin in preventing thrombus formation on mechanical heart valves. Thromb Res. 2010;126(3):e196–200.
Wienen W, Stassen JM, Priepke H, et al. In-vitro profile and ex-vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate. Thromb Haemost. 2007;98(1):155–62.
Markwardt F. Hirudin as alternative anticoagulant: a historical review. Semin Thromb Hemost. 2002;28(5):405–14.
Wienen W, Stassen JM, Priepke H, et al. Effects of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate, on thrombus formation and bleeding time in rats. Thromb Haemost. 2007;98(2):333–8.
Wienen W, Stassen JM, Priepke H, et al. Antithrombotic and anticoagulant effects of the direct thrombin inhibitor dabigatran, and its oral prodrug, dabigatran etexilate, in a rabbit model of venous thrombosis. J Thromb Haemost. 2007;5(6):1237–42.
Stangier J, Rathgen K, Stahle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64(3):292–303.
Troconiz IF, Tillmann C, Liesenfeld KH, et al. Population pharmacokinetic analysis of the new oral thrombin inhibitor dabigatran etexilate (BIBR 1048) in patients undergoing primary elective total hip replacement surgery. J Clin Pharmacol. 2007;47(3):371–82.
Stangier J, Stahle H, Rathgen K, et al. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet. 2008;47(1):47–59.
van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103(6):1116–27.
Ebner T, Wagner K, Wienen W. Dabigatran acylglucuronide, the major human metabolite of dabigatran: in vitro formation, stability, and pharmacological activity. Drug Metab Dispos. 2010;38(9):1567–75.
Stangier J, Stahle H, Rathgen K, et al. Pharmacokinetics and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor, are not affected by moderate hepatic impairment. J Clin Pharmacol. 2008;48(12):1411–9.
Stangier J, Rathgen K, Stahle H, et al. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49(4):259–68.
Stangier J, Eriksson BI, Dahl OE, et al. Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol. 2005;45(5):555–63.
Stangier J, Rathgen K, Stahle H, et al. Coadministration of dabigatran etexilate and atorvastatin: assessment of potential impact on pharmacokinetics and pharmacodynamics. Am J Cardiovasc Drugs. 2009;9(1):59–68.
Clemens A, Haertter S, Friedman J, et al. Twice daily dosing of dabigatran for stroke prevention in atrial fibrillation: a pharmacokinetic justification. Curr Med Res Opin. 2012;28(2):195–201.
Boehringer Ingelheim International GmbH. Pradaxa (dabigatran etexilate) summary of product characteristics. 2008. http://www.eliquis.com/PDF/ELIQUIS%20%C2%AE%20(apixaban)%20SmPC.pdf. Accessed 1 Dec 2012.
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51.
Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91.
Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92.
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363(19):1875–6.
Eikelboom JW, Weitz JI. New anticoagulants. Circulation. 2010;121(13):1523–32.
Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573–9.
Bounameaux H, Reber G. New oral antithrombotics: a need for laboratory monitoring. Against [comment]. J Thromb Haemost. 2010;8(4):627–30.
Weitz JI. New oral anticoagulants in development. Thromb Haemost. 2010;103(1):62–70.
McKeage K. Dabigatran etexilate: a pharmacoeconomic review of its use in the prevention of stroke and systemic embolism in patients with atrial fibrillation. Pharmacoeconomics. 2012;30(9):841–55.
Davidson T, Husberg M, Janzon M, Oldgren J, Levin LA. Cost-effectiveness of dabigatran compared with warfarin for patients with atrial fibrillation in Sweden. Eur Heart J. 2012 [epub ahead of print].
Kamel H, Easton JD, Johnston SC, Kim AS. Cost-effectiveness of apixaban vs warfarin for secondary stroke prevention in atrial fibrillation. Neurology. 2012;79(14):1428–34.
Lee S, Mullin R, Blazawski J, Coleman CI. Cost-effectiveness of apixaban compared with warfarin for stroke prevention in atrial fibrillation. PLoS One. 2012;7(10):e47473.
Hauel NH, Nar H, Priepke H, et al. Structure-based design of novel potent nonpeptide thrombin inhibitors. J Med Chem. 2002;45(9):1757–66.
Acknowledgments
No sources of funding were used to assist in the preparation of this review. The author declares that no conflicts of interest exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scaglione, F. New Oral Anticoagulants: Comparative Pharmacology with Vitamin K Antagonists. Clin Pharmacokinet 52, 69–82 (2013). https://doi.org/10.1007/s40262-012-0030-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-012-0030-9