Abstract
Background and Objective
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are used as adjunctive therapy to lifestyle intervention and metformin treatment in type 2 diabetes mellitus patients, as most GLP-1RAs have cardiovascular benefits; however, a number of adverse events (AEs) have been reported in postmarketing surveillance.
Objective
The aim of this study was to describe the AEs associated with GLP-1RA monotherapy and identify important medical event (IME) signals for GLP-1RAs.
Methods
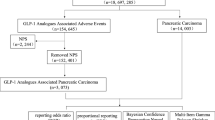
Data from 1 April 2005 to 31 December 2021 from the US FDA Adverse Event Reporting System (FAERS) database were extracted to conduct disproportionality analysis and Bayesian analysis. AEs and IMEs were classified by system organ classes (SOCs) and preferred terms (PTs) according to the Medical Dictionary for Regulatory Activities (MedDRA®). The reporting odds ratio (ROR) and information component (IC) were used to indicate the disproportionality.
Results
A total of 71,515 records involving GLP-1RA monotherapy were submitted to the database, of which 16,350 records were GLP-1RA/IME pairs. Significant disproportionality emerged in five SOCs: ‘gastrointestinal disorders’ (n = 13,104; lower end of the 95% confidence interval (CI) of the IC [IC025] = 1.34), ‘investigations’ (n = 6889; IC025 = 0.64), ‘metabolism and nutrition disorders’ (n = 2943; IC025 = 0.44), ‘neoplasms benign/malignant’ (n = 1989; IC025 = 0.01), and ‘hepatobiliary disorders’ (n = 1497; IC025 = 0.38). The most common AEs were pancreatitis, nausea, and weight decrease. Unexpected significant AEs were detected, such as ileus, osteomyelitis, renal cell carcinoma, nephrolithiasis, and drug-induced liver injury.
Conclusion
The majority of AEs have been listed in the prescribing information or reported in previous studies, however we found significant disproportionality in some specific tumor- and liver-related AEs. Clinicians should pay more attention to the newly detected disproportionality that may be triggered by GLP-1RAs, especially in the vulnerable population after long-term use. Considering the limitations of the FAERS database, there is a need for additional pharmacoepidemiological approaches to validate the results of this study.





Similar content being viewed by others
References
Matheus AS, Tannus LR, Cobas RA, Palma CC, Negrato CA, Gomes MB. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens. 2013;2013: 653789. https://doi.org/10.1155/2013/653789.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–9. https://doi.org/10.2337/dc14-2441.
Honigberg MC, Chang LS, McGuire DK, Plutzky J, Aroda VR, Vaduganathan M. Use of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes and cardiovascular disease: a review. JAMA Cardiol. 2020;5(10):1182–90. https://doi.org/10.1001/jamacardio.2020.1966.
Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39. https://doi.org/10.1056/NEJMoa1612917.
Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57. https://doi.org/10.1056/NEJMoa1509225.
Ma X, Liu Z, Ilyas I, Little PJ, Kamato D, Sahebka A, et al. GLP-1 receptor agonists (GLP-1RAs): cardiovascular actions and therapeutic potential. Int J Biol Sci. 2021;17(8):2050–68. https://doi.org/10.7150/ijbs.59965.
Fujioka K, Harris SR. Barriers and solutions for prescribing obesity pharmacotherapy. Endocrinol Metab Clin North Am. 2020;49(2):303–14. https://doi.org/10.2174/1381612826666200909142126.
Liu C, Zou Y, Qian H. GLP-1R agonists for the treatment of obesity: a patent review (2015-present). Expert Opin Ther Pat. 2020;30(10):781–94. https://doi.org/10.1016/j.ecl.2020.02.007.
Aaseth J, Ellefsen S, Alehagen U, Sundfør TM, Alexander J. Diets and drugs for weight loss and health in obesity-An update. Biomed Pharmacother. 2021;140: 111789. https://doi.org/10.1016/j.biopha.2021.111789.
Wenten M, Gaebler JA, Hussein M, Pelletier EM, Smith DB, Girase P, et al. Relative risk of acute pancreatitis in initiators of exenatide twice daily compared with other anti-diabetic medication: a follow-up study. Diabet Med. 2012;29(11):1412–8. https://doi.org/10.1111/j.1464-5491.2012.03652.x.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44. https://doi.org/10.1056/NEJMoa1607141.
Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141(1):150–6. https://doi.org/10.1053/j.gastro.2011.02.018.
Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–48. https://doi.org/10.1056/NEJMoa1616011.
Monami M, Nreu B, Scatena A, Cresci B, Andreozzi F, Sesti G, et al. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): Data from randomized controlled trials. Diabetes Obes Metab. 2017;19(9):1233–41. https://doi.org/10.1111/dom.12926.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. https://doi.org/10.1056/NEJMoa1603827.
Liao X, Liu Z, Song H. Thyroid dysfunction related to vascular endothelial growth factor receptor tyrosine kinase inhibitors: a real-world study based on FAERS. J Clin Pharm Ther. 2021;46(5):1418–25. https://doi.org/10.1111/jcpt.13472.
Yang R, Yin N, Zhao Y, Li D, Zhang X, Li X, et al. Adverse events during pregnancy associated with entecavir and adefovir: new insights from a real-world analysis of cases reported to FDA adverse event reporting system. Front Pharmacol. 2021;12: 772768. https://doi.org/10.3389/fphar.2021.772768.
Katsuhara Y, Ikeda S. Correlations between SGLT-2 inhibitors and acute renal failure by signal detection using FAERS: stratified analysis for reporting country and concomitant drugs. Clin Drug Investig. 2021;41(3):235–43. https://doi.org/10.1007/s40261-021-01006-9.
Chen C, Chen T, Liang J, Guo X, Xu J, Zheng Y, et al. Cardiotoxicity induced by immune checkpoint inhibitors: a pharmacovigilance study from 2014 to 2019 based on FAERS. Front Pharmacol. 2021;12: 616505. https://doi.org/10.3389/fphar.2021.616505.
Eurpean Medicines Agency. Important medical event list. Available at: https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance/eudravigilance-system-overview. Accessed Jan 2022.
Zhou X, Ye X, Guo X, Liu D, Xu J, Hu F, et al. Safety of SGLT2 inhibitors: a pharmacovigilance study from 2013 to 2021 based on FAERS. Front Pharmacol. 2021;12: 766125. https://doi.org/10.3389/fphar.2021.766125.
Setyawan J, Azimi N, Strand V, Yarur A, Fridman M. Reporting of thromboembolic events with JAK inhibitors: analysis of the FAERS database 2010–2019. Drug Saf. 2021;44(8):889–97. https://doi.org/10.1007/s40264-021-01082-y.
van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11(1):3–10. https://doi.org/10.1002/pds.668.
Björnsson ES. Hepatotoxicity of statins and other lipid-lowering agents. Liver Int. 2017;37(2):173–8. https://doi.org/10.1111/liv.13308.
Jing Y, Yang J, Johnson DB, Moslehi JJ, Han L. Harnessing big data to characterize immune-related adverse events. Nat Rev Clin Oncol. 2022;19(4):269–80. https://doi.org/10.1038/s41571-021-00597-8.
Yu RJ, Krantz MS, Phillips EJ, Stone CA Jr. Emerging causes of drug-induced anaphylaxis: a review of anaphylaxis-associated reports in the FDA adverse event reporting system (FAERS). J Allergy Clin Immunol Pract. 2021;9(2):819-29.e2. https://doi.org/10.1016/j.jaip.2020.09.021.
Yang Z, Yu M, Mei M, Chen C, Lv Y, Xiang L, et al. The association between GLP-1 receptor agonist and diabetic ketoacidosis in the FDA adverse event reporting system. Nutr Metab Cardiovasc Dis. 2022;32(2):504–10. https://doi.org/10.1016/j.numecd.2021.10.003.
Ellenbroek JH, Töns HA, van Westerouen Meeteren MJ, de Graaf N, Hanegraaf MA, Rabelink TJ, et al. Glucagon-like peptide-1 receptor agonist treatment reduces beta cell mass in normoglycaemic mice. Diabetologia. 2013;56(9):1980–6. https://doi.org/10.1007/s00125-013-2957-2.
Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: Are the GLP-1 therapies safe? Diabetes Care. 2013;36(7):2118–25. https://doi.org/10.2337/dc12-2713.
Singh S, Chang HY, Richards TM, Weiner JP, Clark JM, Segal JB. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med. 2013;173(7):534–9. https://doi.org/10.1001/jamainternmed.2013.2720.
Soranna D, Bosetti C, Casula M, Tragni E, Catapano AL, Vecchia CL, et al. Incretin-based drugs and risk of acute pancreatitis: a nested-case control study within a healthcare database. Diabetes Res Clin Pract. 2015;108(2):243–9. https://doi.org/10.1016/j.diabres.2015.02.013.
Faillie JL, Azoulay L, Patenaude V, Hillaire-Buys D, Suissa S. Incretin based drugs and risk of acute pancreatitis in patients with type 2 diabetes: cohort study. BMJ. 2014;348: g2780. https://doi.org/10.1136/bmj.g2780.
Shi J, Deng Q, Wan C, Zheng M, Huang F, Tang B. Fluorometric probing of the lipase level as acute pancreatitis biomarkers based on interfacially controlled aggregation-induced emission (AIE). Chem Sci. 2017;8(9):6188–95. https://doi.org/10.1039/c7sc02189e.
Storgaard H, Cold F, Gluud LL, Vilsbøll T, Knop FK. Glucagon-like peptide-1 receptor agonists and risk of acute pancreatitis in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(6):906–8. https://doi.org/10.1111/dom.12885.
Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18(7):493–502. https://doi.org/10.1038/s41575-021-00457-x.
Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic cancer following acute pancreatitis: a population-based matched cohort study. Am J Gastroenterol. 2018;113(11):1711–9. https://doi.org/10.1038/s41395-018-0255-9.
The Medicines and Healthcare products Regulatory Agency (MHRA). MHRA warns about GLP-1 receptor agonists: reports of diabetic ketoacidosis when concomitant insulin was rapidly reduced or discontinued. 2019. Available at: https://www.gov.uk/drug-safety-update/glp-1-receptor-agonists-reports-of-diabeticketoacidosis-when-concomitant-insulin-was-rapidly-reduced-ordiscontinued. Accessed 22 Dec 2020.
US FDA. FDA is evaluating the need for regulatory action about DKA associated with GLP-1RA. Available at: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/october-december-2019-potential-signals-serious-risksnew-safety-information-identified-fda-adverse. Accessed 22 Dec 2021.
Harrison SA, Gawrieh S, Roberts K, Lisanti CJ, Schwope RB, Cebe KM, et al. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol. 2021;75(2):284–91. https://doi.org/10.1016/j.jhep.2021.02.034.
Kalavalapalli S, Bril F, Guingab J, Vergara A, Garrett TJ, Sunny NE, et al. Impact of exenatide on mitochondrial lipid metabolism in mice with nonalcoholic steatohepatitis. J Endocrinol. 2019;241(3):293–305. https://doi.org/10.1530/JOE-19-0007.
Chan WK, Tan SS, Chan SP, Lee YY, Tee HP, Mahadeva S, et al. Malaysian Society of Gastroenterology and Hepatology consensus statement on metabolic dysfunction-associated fatty liver disease. J Gastroenterol Hepatol. 2022;37(5):795–811. https://doi.org/10.1111/jgh.15787.
Cusi K. Incretin-based therapies for the management of nonalcoholic fatty liver disease in patients with type 2 diabetes. Hepatology. 2019;69(6):2318–22. https://doi.org/10.1002/hep.30670.
US FDA. FDA is evaluating the need for regulatory action about drug-induced liver injury associated with GLP-1RA. Available at: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/january-march-2021-potential-signals-serious-risksnew-safety-information-identified-fda-adverse. Accessed 1 Jan 2022.
Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113–24. https://doi.org/10.1056/NEJMoa2028395.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
No funding was received to conduct this study.
Author contributions
Tingxi Wu and Yang Zhang designed the research, analyzed the data, and wrote a draft of the manuscript. Yanfeng Shi, Shangyi Liu, Kefu Yu and Mei Zhao contributed to the data collection and analysis. Yang Zhang and Zhigang Zhao directed the research and revised the manuscript.
Conflicts of interest
Tingxi Wu, Yang Zhang, Yanfeng Shi, Kefu Yu, Mei Zhao, Shangyi Liu, and Zhigang Zhao have no conflicts of interest to disclose.
Availability of data and material
The data are available in the FAERS (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html).
Ethics approval
No institutional ethics approval was required because this study utilized anonymized data from an open-access database.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, T., Zhang, Y., Shi, Y. et al. Safety of Glucagon-Like Peptide-1 Receptor Agonists: A Real-World Study Based on the US FDA Adverse Event Reporting System Database. Clin Drug Investig 42, 965–975 (2022). https://doi.org/10.1007/s40261-022-01202-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01202-1