Abstract
Background and Objective
Affective disorders account for most cases of suicide. The pharmacological arsenal to treat suicidality is limited and available agents take too long to take effect. A large body of evidence shows optimal results of ketamine for treating depression, but the evidence concerning suicidality has not been fully described. We report the first real-world study of severely depressed patients presenting with suicide ideation who were treated with repeated administration of subcutaneous esketamine.
Methods
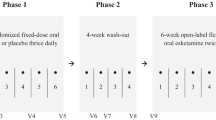
We analyzed data from 70 acutely depressed subjects diagnosed with resistant major depressive disorder or bipolar depression. Subjects were administered subcutaneous esketamine once a week for 6 weeks. The primary efficacy endpoint, the change from baseline to 24-h post-administration 6 in the item 10 Montgomery–Åsberg Depression Rating Scale score, was analyzed using a mixed-effects repeated-measures model.
Results
There were significant effects for time on item 10 Montgomery–Åsberg Depression Rating Scale scores (p < 0.0001) but not for a time × diagnosis interaction (p = 0.164) from baseline to the end of the study. Efficacy of esketamine did not differ between groups (major depressive disorder vs bipolar depression) at any timepoint. Statistical significance on suicidality scores was observed from 24 h after the first administration (p < 0.001), and a further reduction was observed with repeated administrations. Esketamine was safe and well tolerated. Mean heart rate remained stable during the administrations and the blood pressure increase was self-limited.
Conclusions
Repeated subcutaneous esketamine administration had significant anti-suicidality effects in both major depressive disorder and bipolar groups, with a rapid onset of action and a good tolerability profile. Large randomized controlled trials are warranted to confirm these preliminary findings.

Similar content being viewed by others
References
Xu J, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: final data for 2007. Natl Vital Stat Rep. 2010;58(19):1–19.
Heron M. Deaths: leading causes for 2008. Natl Vital Stat Rep. 2012;60(6):1–94.
Kessler RC. The costs of depression. Psychiatr Clin North Am. 2012;35(1):1–14.
World Health Organization. Suicide worldwide in 2019. 2021; p. 1–35. https://www.who.int/publications/i/item/9789240026643. Accessed 26 Jul 2022.
National Institute of Mental Health. Suicide. 2022. https://www.nimh.nih.gov/health/statistics/suicide. Accessed 26 Jul 2022.
Klonsky ED, May AM. The three-step theory (3ST): a new theory of suicide rooted in the “ideation-to-action” framework. Int J Cogn Ther. 2015;8(2):114–29.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: American Psychiatric Publishing; 2013.
Arsenault-Lapierre G, Kim C, Turecki G. Psychiatric diagnoses in 3275 suicides: a meta-analysis. BMC Psychiatry. 2004;4:1–11.
Pompili M, Gonda X, Serafini G, Innamorati M, Sher L, Amore M, et al. Epidemiology of suicide in bipolar disorders: a systematic review of the literature. Bipolar Disord. 2013;15(5):457–90.
Kessing LV, Søndergård L, Kvist K, Andersen PK. Suicide risk in patients treated with lithium. Arch Gen Psychiatry. 2005;62(8):860–6.
Wasserman D, Rihmer Z, Rujescu D, Sarchiapone M, Sokolowski M, Titelman D, et al. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry. 2012;27(2):129–41.
Jacobs DG, Baldessarini RJ, Conwell Y, Fawcett JA, Horton L, Meltzer H, et al. Assessment and treatment of patients with suicidal behaviors. APA practice guidelines. Washington, DC: American Psychiatric Publishing; 2010.
Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8(5 Pt2):625–39.
Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346: f3646.
Oquendo MA, Galfalvy HC, Currier D, Grunebaum MF, Sher L, Sullivan GM, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168(10):1050–6.
Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A, et al. Clozapine treatment for suicidality in schizophrenia: international suicide prevention trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82–91.
Modestin J, Dal Pian D, Agarwalla P. Clozapine diminishes suicidal behavior: a retrospective evaluation of clinical records. J Clin Psychiatry. 2005;66(4):534–8.
Fink M, Kellner CH, McCall WV. The role of ECT in suicide prevention. J ECT. 2014;30(1):5–9.
Price RB, Mathew SJ. Does ketamine have anti-suicidal properties? Current status and future directions. CNS Drugs. 2015;29(3):181–8.
Hoyer C, Kranaster L, Janke C, Sartorius A. Impact of the anesthetic agents ketamine, etomidate, thiopental, and propofol on seizure parameters and seizure quality in electroconvulsive therapy: a retrospective study. Eur Arch Psychiatry Clin Neurosci. 2014;264(3):255–61.
Elliott E, Hanid TK, Arthur LJ, Kay B. Ketamine anaesthesia for medical procedures in children. Arch Dis Child. 1976;51(1):56–9.
Salvadore G, Singh JB. Ketamine as a fast acting antidepressant: current knowledge and open questions. CNS Neurosci Ther. 2013;19(6):428–36.
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–4.
Aan Het Rot M, Zarate Jr CA, Charney DS, Mathew SJ. Ketamine for depression: where do we go from here? Biol Psychiatry. 2012;72(7):537–47.
Mathew SJ, Shah A, Lapidus K, Clark C, Jarun N, Ostermeyer B, et al. Ketamine for treatment-resistant unipolar depression. CNS Drugs. 2012;26(3):189–204.
Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71(11):939–46.
Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134–42.
Grunebaum MF, Galfalvy HC, Choo T-H, Keilp JG, Moitra VK, Parris MS, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327–35.
Grunebaum MF, Ellis SP, Keilp JG, Moitra VK, Cooper TB, Marver JE, et al. Ketamine versus midazolam in bipolar depression with suicidal thoughts: a pilot midazolam-controlled randomized clinical trial. Bipolar Disord. 2017;19(3):176–83.
Ballard ED, Ionescu DF, Vande Voort JL, Niciu MJ, Richards EM, Luckenbaugh DA, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161–6.
Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150–8.
Reinstatler L, Youssef NA. Ketamine as a potential treatment for suicidal ideation: a systematic review of the literature. Drugs R D. 2015;15(1):37–43.
Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14(8):1127–31.
Loo CK, Gálvez V, O’keefe E, Mitchell PB, Hadzi-Pavlovic D, Leyden J, et al. Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand. 2016;134(1):48–56.
Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620–30.
Fu DJ, Ionescu DF, Li X, Lane R, Lim P, Sanacora G, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry. 2020;81(3):19m13191.
Ionescu DF, Fu DJ, Qiu X, Lane R, Lim P, Kasper S, et al. Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II). Int J Neuropsychopharmacol. 2021;24(1):22–31.
McIntyre RS, Rodrigues NB, Lee Y, Lipsitz O, Subramaniapillai M, Gill H, et al. The effectiveness of repeated intravenous ketamine on depressive symptoms, suicidal ideation and functional disability in adults with major depressive disorder and bipolar disorder: results from the Canadian Rapid Treatment Center of Excellence. J Affect Disord. 2020;274:903–10.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33.
Fountoulakis KN, Yatham LN, Grunze H, Vieta E, Young AH, Blier P, et al. The CINP guidelines on the definition and evidence-based interventions for treatment-resistant bipolar disorder. Int J Neuropsychopharmacol. 2020;23(4):230–56.
Gaynes BN, Lux L, Gartlehner G, Asher G, Forman-Hoffman V, Green J, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134–45.
Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9.
Fekadu A, Wooderson S, Donaldson C, Markopoulou K, Masterson B, Poon L, et al. A multidimensional tool to quantify treatment resistance in depression: the Maudsley Staging Method. J Clin Psychiatry. 2009;70(2):177–84.
Del Sant LC, Sarin LM, Magalhães EJM, Lucchese AC, Tuena MA, Nakahira C, et al. Effects of subcutaneous esketamine on blood pressure and heart rate in treatment-resistant depression. J Psychopharmacol. 2020;341(10):1155–62.
Xiong J, Lipsitz O, Chen-Li D, Rosenblat JD, Rodrigues NB, Carvalho I, et al. The acute antisuicidal effects of single-dose intravenous ketamine and intranasal esketamine in individuals with major depression and bipolar disorders: a systematic review and meta-analysis. J Psychiatr Res. 2021;134:57–68.
Li D-J, Wang F-C, Chu C-S, Chen T-Y, Tang C-H, Yang W-C, et al. Significant treatment effect of add-on ketamine anesthesia in electroconvulsive therapy in depressive patients: a meta-analysis. Eur Neuropsychopharmacol. 2017;27(1):29–41.
Li X-M, Shi Z-M, Wang P-J, Hu H. Effects of ketamine in electroconvulsive therapy for major depressive disorder: meta-analysis of randomised controlled trials. Gen Psychiatry. 2020;33(3): e100117.
Lengvenyte A, Olié E, Courtet P. Suicide has many faces, so does ketamine: a narrative review on ketamine’s antisuicidal actions. Curr Psychiatry Rep. 2019;21(12):132.
Ionescu DF, Felicione JM, Gosai A, Cusin C, Shin P, Shapero BG, et al. Ketamine-associated brain changes: a review of the neuroimaging literature. Harv Rev Psychiatry. 2018;26(6):320–39.
Duman RS, Deyama S, Fogaça MV. Role of BDNF in the pathophysiology and treatment of depression: activity-dependent effects distinguish rapid-acting antidepressants. Eur J Neurosci. 2021;53(1):126–39.
Haroon E, Fleischer C, Chen X, Felger J, Woolwine B, Hu X, et al. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Neuropsychopharmacology. 2015;21(10):1351–7.
Rizk MM, Galfalvy H, Singh T, Keilp JG, Sublette ME, Oquendo MA, et al. Toward subtyping of suicidality: brief suicidal ideation is associated with greater stress response. J Affect Disord. 2018;230:87–92.
Vichaya EG, Dantzer R. Inflammation-induced motivational changes: perspective gained by evaluating positive and negative valence systems. Curr Opin Behav Sci. 2018;22:90–5.
Short B, Dong V, Gálvez V, Vulovic V, Martin D, Bayes AJ, et al. Development of the Ketamine Side Effect Tool (KSET). J Affect Disord. 2020;266:615–20.
Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiat. 2018;75:139–48.
Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiat. 2019;76:893–903.
Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176:428–38.
Abuhelwa AY, Somogyi AA, Loo CK, Glue P, Barratt DT, Foster DJR. Population pharmacokinetics and pharmacodynamics of the therapeutic and adverse effects of ketamine in patients with treatment-refractory depression. Clin Pharmacol Ther. 2022. https://doi.org/10.1002/cpt.2640.
Clements JA, Nimmo WS, Grant IS. Bioavailability, pharmacokinetics, and analgesic activity of ketamine in humans. J Pharm Sci. 1982;71:539–42.
Acknowledgements
The authors express their gratitude to the participants in this study and to the staff of PRODAF - Programa de Transtornos Afetivos, Federal University of Sao Paulo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). The funding institutions played no role in the study design, data collection, statistical analysis, or data interpretation, in the manuscript writing, or in the decision to submit the article for publication.
Conflicts of interest
Acioly Luiz Tavares Lacerda has received consulting fees from Hoffmann-La Roche, Genentech, Janssen Pharmaceutical, Daiichi Sankyo, Cristalia Produtos Químicos e Farmacêuticos, Pfizer, Mantecorp Indústria Química e Farmacêutica, Libbs Farmacêutica, FQM Farma, and Sanofi-Aventis over the last 24 months and has received research fees from Janssen Pharmaceutical, Eli Lilly, H. Lundbeck A/S, Servier Laboratories, Hoffman-La Roche, FQM Farma, and Forum Pharmaceuticals. Juliana Surjan reports personal fees from Cristalia Produtos Químicos e Farmacêuticos and Abbot and non-financial support from Janssen Pharmaceutical, outside the submitted work. Carolina Nakahira reports non-financial support from Eurofarma, Sanofi-Aventis, and Cristalia Produtos Quimicos e Farmaceuticos, outside the submitted work. Raphael de Oliveira Cerqueira reports personal fees from Janssen Pharmaceutical and Abbot, outside the submitted work. Luciana Maria Sarin reports personal fees from Daiichi Sankyo Brasil, Lundbeck A/S, Pfizer, and Janssen Pharmaceutical, and non-financial support from Takeda Brasil, Moksha8 Brasil, and Torrent Pharma, outside the submitted work. Eduardo Magalhães reports non-financial support from Torrent Pharma and Hypera Pharma, outside the submitted work. José Alberto Del Porto reports being an active speaker and board advisor at Daiichi Sankyo Brasil, Pfizer, Janssen Pharmaceutical, Cristalia Produtos Quimicos e Farmaceuticos, Libbs Farmaceutica, H. Lundbeck A/S, Wyeth, Ache Laboratorios Farmaceuticos, and Eurofarma. The remaining authors declare no potential conflicts of interest.
Ethics approval
The study was approved by the local institutional review board (no. 3.115.329). All procedures in this study were in accordance with the 1964 Declaration of Helsinki.
Consent to participate
All participants signed the approved version of the written informed consent before performing any procedure related to the study. Patients were asked to attend all treatment sessions accompanied by a responsible adult. All interviews were conducted by trained psychiatrists. Data were inserted into a databank using the RedCap platform.
Consent for publication
Not applicable.
Availability of data and material
The datasets analyzed in the current study are not publicly available because of local policies.
Code availability
Not applicable.
Author contributions
ALTL designed the study, wrote the protocol, undertook statistical analyses, and revised the manuscript. JS wrote the manuscript, participated in the data collection, and interpreted the results. JDG helped write the manuscript and analyze the results. JADP helped design the study and implement the study’s research unit. LMS helped design the study, write the protocol, and implement the study’s research unit. RSD participated in the data collection and helped extract data from the bank. ROC helped write the manuscript. EM, LCDS, ACL, MAT, CN, VARF, MSS, and MGB participated in the data collection.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Surjan, J., Grossi, J.D., Del Porto, J.A. et al. Efficacy and Safety of Subcutaneous Esketamine in the Treatment of Suicidality in Major Depressive Disorder and Bipolar Depression. Clin Drug Investig 42, 865–873 (2022). https://doi.org/10.1007/s40261-022-01193-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01193-z