Abstract
Background and Objective
Biologic disease-modifying anti-rheumatic drugs (bDMARDs) are used either when conventional synthetic DMARDs are ineffective or when disease activity is high and with poor prognostic factors, based on various clinical guidelines. The purpose of this study was to investigate the prescribing trends of bDMARDs for patients with rheumatoid arthritis in Japan, and to clarify whether the pharmacological therapy of bDMARDs is administered based on guidelines.
Methods
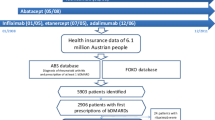
We conducted a descriptive epidemiological study from 2012 to 2018 using the JMDC Claims Database, a nationwide claims database, and described the annual changes based on the number of patients prescribed bDMARDs. Anti-rheumatic drugs were identified based on the Anatomical Therapeutic Chemical codes, including methotrexate, glucocorticoids, non-steroidal anti-inflammatory drugs and bDMARDs.
Results
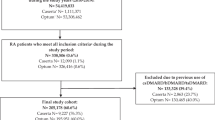
From the database including 6,862,244 people, the data of 6407 patients with rheumatoid arthritis were extracted. The present study demonstrated that the proportion of patients prescribed bDMARDs was 1.0 per 1000 people, with those aged ≥ 65 years being the most common age group. The proportion of patients with rheumatoid arthritis who were prescribed bDMARDs increased significantly over time (p < 0.0001). Additionally, the concomitant proportions of methotrexate (p < 0.0001), non-steroidal anti-inflammatory drugs (p < 0.0001) and glucocorticoids (p = 0.0001) prescribed with bDMARDs decreased significantly over time.
Conclusions
The increase in bDMARD monotherapy may be attributed to the new bDMARDs that have been launched sequentially; furthermore, physicians have come to recognise monotherapy as the mainstay of treatment. Future studies must accumulate evidence on the long-term efficacy and safety of bDMARDs.




Similar content being viewed by others
References
Gibofsky A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: a synopsis. Am J Manag Care. 2014;20(7 Suppl.):S128–35.
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38. https://doi.org/10.1016/s0140-6736(16)30173-8.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. https://doi.org/10.1002/art.27584.
Smyth CJ. Optimum therapeutic program in seropositive nodular rheumatoid arthritis. Med Clin N Am. 1968;52(3):687–98.
Köhler BM, Günther J, Kaudewitz D, Lorenz HM. Current therapeutic options in the treatment of rheumatoid arthritis. J Clin Med. 2019;8(7):938. https://doi.org/10.3390/jcm8070938.
Nell VP, Machold KP, Eberl G, Stamm TA, Uffmann M, Smolen JS. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology (Oxford). 2004;43(7):906–14. https://doi.org/10.1093/rheumatology/keh199.
van Dongen H, van Aken J, Lard LR, Visser K, Ronday HK, Hulsmans HM, et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2007;56(5):1424–32. https://doi.org/10.1002/art.22525.
Smolen JS, Landewé R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69(6):964–75. https://doi.org/10.1136/ard.2009.126532.
Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(5):625–39. https://doi.org/10.1002/acr.21641.
Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344(12):907–16. https://doi.org/10.1056/nejm200103223441207.
Rein P, Mueller RB. Treatment with biologicals in rheumatoid arthritis: an overview. Rheumatol Ther. 2017;4(2):247–61. https://doi.org/10.1007/s40744-017-0073-3.
Dahabreh IJ, Kent DM. Can the learning health care system be educated with observational data? JAMA. 2014;312(2):129–30. https://doi.org/10.1001/jama.2014.4364.
Camm AJ, Fox KAA. Strengths and weaknesses of “real-world” studies involving non-vitamin K antagonist oral anticoagulants. Open Heart. 2018;5(1):e000788. https://doi.org/10.1136/openhrt-2018-000788.
Yamanaka H, Sugiyama N, Inoue E, Taniguchi A, Momohara S. Estimates of the prevalence of and current treatment practices for rheumatoid arthritis in Japan using reimbursement data from health insurance societies and the IORRA cohort (I). Mod Rheumatol. 2014;24(1):33–40. https://doi.org/10.3109/14397595.2013.854059.
Katada H, Yukawa N, Urushihara H, Tanaka S, Mimori T, Kawakami K. Prescription patterns and trends in anti-rheumatic drug use based on a large-scale claims database in Japan. Clin Rheumatol. 2015;34(5):949–56. https://doi.org/10.1007/s10067-013-2482-1.
Sullivan E, Kershaw J, Blackburn S, Choi J, Curtis JR, Boklage S. Biologic disease-modifying antirheumatic drug prescription patterns for rheumatoid arthritis among United States physicians. Rheumatol Ther. 2020;7(2):383–400. https://doi.org/10.1007/s40744-020-00203-w.
Sullivan E, Kershaw J, Blackburn S, Mahajan P, Boklage SH. Biologic disease-modifying antirheumatic drug prescription patterns among rheumatologists in Europe and Japan. Rheumatol Ther. 2020;7(3):517–35. https://doi.org/10.1007/s40744-020-00211-w.
Yamanaka H, Tanaka E, Nakajima A, Furuya T, Ikari K, Taniguchi A, et al. A large observational cohort study of rheumatoid arthritis, IORRA: providing context for today’s treatment options. Mod Rheumatol. 2020;30(1):1–6. https://doi.org/10.1080/14397595.2019.1660028.
Fassmer AM, Garbe E, Schmedt N. Frequency and trends of disease-modifying antirheumatic drug (DMARD) use in Germany. Pharmacol Res Perspect. 2016;4(5):e00254. https://doi.org/10.1002/prp2.254.
Yazici Y, Shi N, John A. Utilization of biologic agents in rheumatoid arthritis in the United States: analysis of prescribing patterns in 16,752 newly diagnosed patients and patients new to biologic therapy. Bull NYU Hosp Jt Dis. 2008;66(2):77–85.
de Thurah A, Nørgaard M, Johansen M, Stengaard-Pedersen K. Time to methotrexate treatment in patients with rheumatoid arthritis referred to hospital. Scand J Rheumatol. 2010;39(1):19–25. https://doi.org/10.3109/03009740903185987.
Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69(1):88–96. https://doi.org/10.1136/ard.2008.105197.
Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381(9877):1541–50. https://doi.org/10.1016/s0140-6736(13)60250-0.
Tarp S, Furst DE, Dossing A, Østergaard M, Lorenzen T, Hansen MS, et al. Defining the optimal biological monotherapy in rheumatoid arthritis: a systematic review and meta-analysis of randomised trials. Semin Arthritis Rheum. 2017;46(6):699–708. https://doi.org/10.1016/j.semarthrit.2016.09.003.
Tarp S, Jørgensen TS, Furst DE, Dossing A, Taylor PC, Choy EH, et al. Added value of combining methotrexate with a biological agent compared to biological monotherapy in rheumatoid arthritis patients: a systematic review and meta-analysis of randomised trials. Semin Arthritis Rheum. 2019;48(6):958–66. https://doi.org/10.1016/j.semarthrit.2018.10.002.
Emery P, Pope JE, Kruger K, Lippe R, DeMasi R, Lula S, et al. Efficacy of monotherapy with biologics and JAK inhibitors for the treatment of rheumatoid arthritis: a systematic review. Adv Ther. 2018;35(10):1535–63. https://doi.org/10.1007/s12325-018-0757-2.
Doria A, Zavaglia D. Monotherapy is a relevant option in rheumatoid arthritis treatment: a literature review. Clin Exp Rheumatol. 2019;37(5):862–71.
Hyrich KL, Symmons DP, Watson KD, Silman AJ. Comparison of the response to infliximab or etanercept monotherapy with the response to cotherapy with methotrexate or another disease-modifying antirheumatic drug in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54(6):1786–94. https://doi.org/10.1002/art.21830.
Attar SM. Adverse effects of low dose methotrexate in rheumatoid arthritis patients: a hospital-based study. Saudi Med J. 2010;31(8):909–15.
Gilani ST, Khan DA, Khan FA, Ahmed M. Adverse effects of low dose methotrexate in rheumatoid arthritis patients. J Coll Physicians Surg Pak. 2012;22(2):101–4.
Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem. 2018;158:502–16. https://doi.org/10.1016/j.ejmech.2018.09.027.
Capell HA, Madhok R, Hunter JA, Porter D, Morrison E, Larkin J, et al. Lack of radiological and clinical benefit over two years of low dose prednisolone for rheumatoid arthritis: results of a randomised controlled trial. Ann Rheum Dis. 2004;63(7):797–803. https://doi.org/10.1136/ard.2003.014050.
Morrison E, Capell HA. Corticosteroids in the management of early and established rheumatoid disease. Rheumatology (Oxford). 2006;45(9):1058–61. https://doi.org/10.1093/rheumatology/kel230.
Iikuni N, Inoue E, Tanaka E, Hara M, Tomatsu T, Kamatani N, et al. Low disease activity state with corticosteroid may not represent “true” low disease activity state in patients with rheumatoid arthritis. Rheumatology (Oxford). 2008;47(4):519–21. https://doi.org/10.1093/rheumatology/ken047.
Tanaka E, Mannalithara A, Inoue E, Iikuni N, Taniguchi A, Momohara S, et al. Effects of long-term corticosteroid usage on functional disability in patients with early rheumatoid arthritis, regardless of controlled disease activity. Rheumatol Int. 2012;32(3):749–57. https://doi.org/10.1007/s00296-010-1638-4.
Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48(1):35–45. https://doi.org/10.1002/art.10697.
Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400–11. https://doi.org/10.1002/art.20217.
Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50(4):1051–65. https://doi.org/10.1002/art.20159.
van der Heijde D, Klareskog L, Rodriguez-Valverde V, Codreanu C, Bolosiu H, Melo-Gomes J, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54(4):1063–74. https://doi.org/10.1002/art.21655.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interest
Atsushi Hirata, Ryosuke Ota, Takeo Hata, Takeshi Hamada, Masami Nishihara, Kazuhisa Uchiyama and Takahiro Katsumata have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
All procedures in this study involving human participants were performed in accordance with the ethical standards of the institutional research committee, and with the 1964 Declaration of Helsinki, its later amendments and comparable ethical standards. This study was approved by the Ethics Committee of Osaka Medical and Pharmaceutical University (Approval ID: RIN-41, 2762) on 6 August, 2019.
Consent to participate
This study used anonymised information from the JMDC Claims Database; therefore, in accordance with the ethical guidelines for medical and health research involving human subjects in Japan, informed consent was not required.
Consent for publication
Not applicable.
Availability of data and material
The data that support the findings of this study are available from JMDC Inc. but were used under license for the current study; therefore, restrictions apply and the data are not publicly available. For inquiries about access to the dataset used in this study, please contact JMDC (https://www.jmdc.co.jp).
Code availability
Not applicable.
Authors’ contributions
Atsushi Hirata: conceptualisation, methodology, validation, formal analysis, investigation, writing—original draft. Ryosuke Ota: conceptualisation, methodology, validation, writing—review and editing. Takeo Hata: conceptualisation, methodology, validation, formal analysis, writing—review and editing, supervision. Takeshi Hamada: writing—review and editing. Masami Nishihara: writing—review and editing. Kazuhisa Uchiyama: writing—review and editing. Takahiro Katsumata; writing—review and editing, project administration.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hirata, A., Ota, R., Hata, T. et al. Prescribing Trends of Biologic Disease-Modifying Anti-rheumatic Drugs Using a Claims Database from 6 Million People in Japan. Clin Drug Investig 41, 967–974 (2021). https://doi.org/10.1007/s40261-021-01082-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-021-01082-x