Abstract
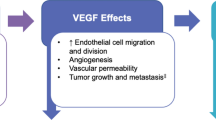
FKB238 is a biosimilar of bevacizumab (a monoclonal antibody against vascular endothelial growth factor) approved for use in the same types of cancer as reference bevacizumab. FKB238 has similar physicochemical and pharmacodynamic properties to those of reference bevacizumab and pharmacokinetic similarity was shown in healthy volunteers and in patients with non-small cell lung cancer (NSCLC). FKB238 demonstrated equivalent clinical efficacy to reference bevacizumab in patients with advanced or recurrent nonsquamous NSCLC, with similar tolerability, safety and immunogenicity profiles.

Similar content being viewed by others
References
European Medicines Agency. Equidacent: CHMP assessment report. 2020. https://www.ema.europa.eu/en/documents/assessment-report/equidacent-epar-public-assessment-report_en.pdf. Accessed 19 Jul 2021
Kaito H, van den Berg F, Niewiarowski A, et al. A randomized, double-blind, single dose comparison of pharmacokinetics and safety of FKB238 with bevacizumab [abstract no. e14007]. J Clin Oncol. 2017;35(Suppl 15).
Syrigos K, Abert I, Andric Z, et al. Efficacy and safety of bevacizumab biosimilar FKB238 versus originator bevacizumab: results from AVANA, a phase III trial in patients with non-squamous non-small-cell lung cancer (non-sq-NSCLC). BioDrugs. 2021. https://doi.org/10.1007/s40259-021-00489-4.
European Medicines Agency. Equidacent (bevacizumab): summary of product characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/equidacent-epar-product-information_en.pdf. Accessed 19 Jul 2021
Acknowledgements
During the peer review process the manufacturer of FKB238 was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Yahiya Y. Syed is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: K. Araki, Department of Medical Oncology, Gunma Prefectural Cancer Center, Ohta, Gunma, Japan; P. Gascon, Laboratory of Molecular and Translational Oncology, CELLEX, Barcelona, Spain.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Syed, Y.Y. FKB238: A Bevacizumab Biosimilar. Clin Drug Investig 41, 825–828 (2021). https://doi.org/10.1007/s40261-021-01065-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-021-01065-y