Abstract
Background and objectives
The new filgrastim formulation, BK0023, whose synthesis method is patented, was tested in a phase I clinical study that was aimed at investigating the pharmacodynamic and pharmacokinetic equivalence and the safety of BK0023 in healthy male subjects.
Methods
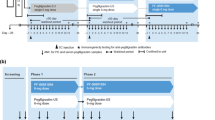
Single and multiple escalating doses were administered to healthy male volunteers according to a double-blind, randomised, two-way crossover design. Thirty-two subjects received subcutaneous filgrastim 2.5 µg/kg/day for 7 consecutive days in each period, 36 subjects received 5 µg/kg/day for 7 days in each period, and 22 subjects received 10 µg/kg/day for 5 days. Absolute neutrophil count (ANC) and CD34+ cell count were measured in whole blood as primary and secondary pharmacodynamic parameters. Filgrastim concentrations were measured in serum to calculate the primary pharmacokinetic parameters.
Results
The maximum ANC and the area under the curve of the ANC after the first dose and to the end of treatment satisfied the equivalence criterion (95 % confidence intervals within 85–115 or 85–117 % in case of log-transformation). At all three dose regimens, BK0023 was also bioequivalent to the reference product in terms of pharmacokinetic profile of serum filgrastim. The frequency of the treatment-emergent adverse events did not differ significantly between treatments, with the most frequent untoward effects being back and bone pain.
Conclusions
Equivalence could be established using both the baseline-adjusted values and the original unadjusted values. The tested formulation at all three dose regimens was also bioequivalent to the reference product in terms of pharmacokinetic profile.



Similar content being viewed by others
References
Skipper HE. Kinetics of mammary tumour cell growth and implications for therapy. Cancer. 1971;28(6):1479–99.
Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G. Filgrastim (r-metHuG-CSF) the first 10 years. Blood. 1996;88(6):1907–29.
Simmers RN, Webber LM, Shannon MF, Garson OM, Wong G, Vadas MA, et al. Localization of the G-CSF gene on chromosome 17 proximal to the breakpoint in the t (15;17) in acute promyelocytic leukemia. Blood. 1987;70(1):330–2.
Nomura H, Imazeki I, Oheda M, Kubota N, Tamura M, Ono M, et al. Purification and characterization of human granulocyte colony-stimulating factor (G-CSF). EMBO J. 1986;5(5):871–6.
Nicola NA, Metcalf D, Matsumoto M, Johnson GR. Purification of a factor inducing differentiation in murine myelomonocytic leukaemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983;258(14):9017–23.
Nicola NA, Begley CG, Metcalf D. Identification of the human analogue of a regulator that induces differentiation in murine leukaemic cells. Nature. 1985;314(6012):625–8.
Fukunaga R, Ishizaka-Ikeda E, Nagata S. Purification and characterization of the receptor for murine granulocyte colony—stimulating factor. J Biol Chem. 1990;265(23):14008–15.
Layton JE, Hall NE, Connell F, Venhorst J, Treutlein HR. Identification of ligand-binding site III on the immunoglobulin-like domain of the granulocyte colony-stimulating factor receptor. J Biol Chem. 2001;276:36779–87.
Nicola NA. Hemopoietic cell growth factors and their receptors. Annu Rev Biochem. 1989;58:45–77.
Nagata S, Fukunaga R. Granulocyte colony-stimulating factor and its receptor. Prog Growth Factor Res. 1991;3(2):131–41.
Shimoda K, Okamura S, Harada N, Kondo S, Okamura T, Niho Y. Identification of a functional receptor for granulocyte colony-stimulating factor on platelets. J Clin Invest. 1993;91(4):1310–3.
Boneberg EM, Hareng L, Gantner F, Wendel A, Hartung T. Human monocytes express functional receptors for granulocyte colony-stimulating factor that mediate suppression of monokines and interferon-γ. Blood. 2000;95(1):270–6.
Bussolino F, Wang JM, Defilippi P, Turrini F, Sanavio F, Edgell CS, et al. Granulocyte- and granulocyte-macrophage colony-stimulating factors induce human endothelial cells to migrate and proliferate. Nature. 1989;337:471–3.
Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–808.
Welte K, Platzer E, Lu L, Gabrilove JL, Levi E, Mertelsmann R, et al. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci USA. 1985;82:1526–30.
Souza LM, Boone TC, Gabriolove J, Lai PH, Zsebo KM, Murdock DC, et al. Recombinant human granulocyte colony stimulating factor: effect on normal and leukemic myeloid cells. Science. 1986;232:61–5.
Zsebo KM, Cohen AM, Murdock DC, Boone TC, Inoue H, Chazin VR, et al. Recombinant human granulocyte colony stimulating factor: molecular and biological characterization. Immunobiology. 1986;172:175–84.
Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, et al. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med. 2005;11(3):305–11.
Bönig H, Silbermann S, Weller S, Kirschke R, Körholz D, Janssen G, et al. Glycosylated vs non-glycosylated granulocyte colony-stimulating factor (G-CSF)—results of a prospective randomised monocentre study. Bone Marrow Transplant. 2001;28:259–64.
Crobu D, Spinetti G, Schrepfer R, Tonon G, Saccani Jotti G, Onali P, et al. Preclinical and clinical phase I studies of a new recombinant filgrastim (BK0023) in comparison with Neupogen®. BMC Pharmacol Toxicol. doi:10.1186/2050-6511-15-7 (Epub 21 Feb 2014).
Vanoni M, Tortora P, Tonon G, Taylor J, Orsini G. Inventors. Bio-ker S.r.l., assignee. US patent 20030186258 A1, 2 Oct 2003.
van der Auwera P, Platzer E, Xu ZX, Schulz R, Feugeas O, Capdeville R, et al. Pharmacodynamics and pharmacokinetics of single doses of subcutaneous pegylated human G-CSF mutant (Ro 25-8315) in healthy volunteers: comparison with single and multiple daily doses of filgrastim. Am J Hematol. 2001;66(4):245–51.
The European Agency for the Evaluation of Medicinal Products (EMEA). Note for guidance on the investigation of bioavailability and bioequivalence. Guideline CPMP/EWP/QWP/1401/98; London, 26 Jul 2001.
The European Agency for the Evaluation of Medicinal Products (EMEA). Annex to guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. Guidance on similar medicinal products containing recombinant granulocyte-colony stimulating factor. Guideline CHMP/BMWP/31329/2005; London, 22 Feb 2006.
Tanaka R, Matsudaira T, Aizawa J, Ebihara Y, Muraoka K, Tsuji K, et al. Characterization of peripheral blood progenitor cells (PBPC) mobilized by filgrastim (rHuG-CSF) in normal volunteers: dose-effect relationship for filgrastim with the character of mobilized PBPC. Br J Haematol. 1996;92(4):795–803.
Borleffs JCC, Bosschaert M, Vrehen HM, Schneider MME, van Strijp J, Small MK, et al. Effect of escalating doses of recombinant human granulocyte colony-stimulating factor (filgrastim) on circulating neutrophils in healthy subjects. Clin Ther. 1998;20(4):722–36.
Layton JE, Hockman H, Sheridan WP, Morstyn G. Evidence for a novel in vivo control mechanism of granulopoiesis: mature cell-related control of a regulatory growth factor. Blood. 1989;74:1303–7.
Young JD, Cheung EN, Tanaka H, Hasibeder H, Asano K, Shimosaka A. Bioavailability of subcutaneously administered non-glycosylated recombinant hG-CSF (Filgrastim) in normal and neutropenic rats and in humans. Proc Am Soc Clin Oncol. 1994;13:162.
Acknowledgments
The sponsor reviewed and approved the study design, and reviewed and approved the analysis and interpretation of data. Bio-Ker S.r.l. reviewed and approved the manuscript for publication.
Antonio Rusca and Milko M. Radicioni reviewed and approved the study design, were responsible for the clinical activities, and collected the data; Luca Loprete performed the pharmacodynamics and pharmacokinetic analysis; Domenica M. G. Lamparelli was responsible for the bioanalysis of ANC and CD34+ cells; Jutta Michael Hepp was responsible for the bioanalysis of filgrastim; Giancarlo Tonon, Davide Crobu and Rodolfo Schrepfer reviewed and approved the design of the study and the draft manuscript; Gaia Spinetti was involved in the development of the clinical study; and Andrea F. D. Di Stefano managed the study co-ordination, wrote the clinical trial report and drafted the manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Financial support for this project was received from Bio-Ker S.r.l., Italy. The relationships between the sponsor, Bio-Ker S.r.l. and CROSS Research S.A., Accelera S.r.l., Nuvisan GmbH and IRCCS MultiMedica were regulated by financial agreements.
Conflicts of interest
Antonio Rusca, Milko M. Radicioni, Luca Loprete, Domenica M. G. Lamparelli, Jutta Michael Hepp and Andrea F. D. Di Stefano are employees of CROSS Research S.A., Accelera S.r.l. and Nuvisan GmbH, whose relationships with the sponsor were regulated by financial agreements. Giancarlo Tonon, Davide Crobu and Rodolfo Schrepfer are employees of Bio-ker S.r.l., and Gaia Spinetti is an employee of IRCCS MultiMedica; both companies are owned by MultiMedica Holding.
Ethical approval
The study was conducted in compliance with the Swiss ordinance on clinical trials of therapeutic agents and in accordance with the Declaration of Helsinki and the general principles of ICH Harmonised Tripartite Guidelines for GCP, and after approval by the competent Ethics Committee of Canton Ticino, Switzerland.
Informed consent
Written informed consent was received from all study subjects prior to undergoing any study procedure.
Additional information
Registered at ClinicalTrials.gov on 3 September 2013 (identifier NCT01933971).
Rights and permissions
About this article
Cite this article
Di Stefano, A.F.D., Spinetti, G., Rusca, A. et al. Recombinant Filgrastim (BK0023) Pharmacodynamics and Pharmacokinetics After Single and Multiple Escalating Doses in an Equivalence Study in Healthy Men. Clin Drug Investig 35, 533–545 (2015). https://doi.org/10.1007/s40261-015-0310-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-015-0310-x